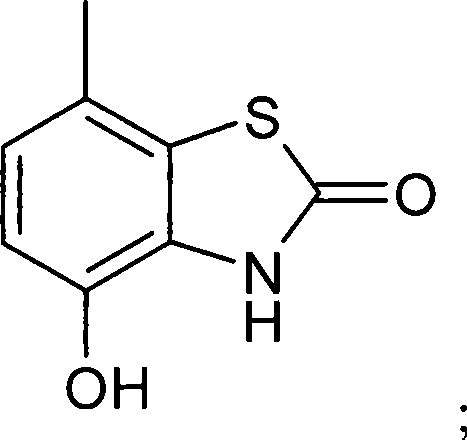
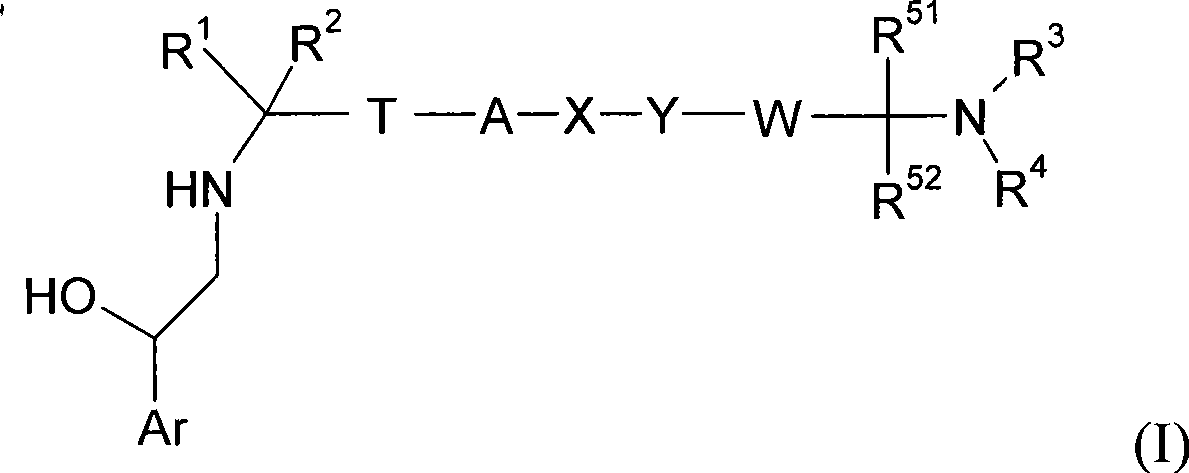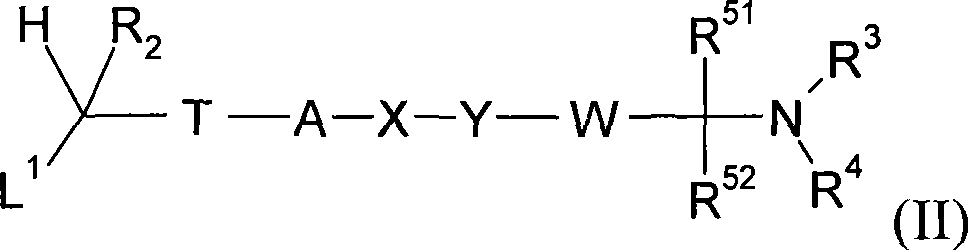Bezothiazol derivatives as beta2 adrenoreceptor agonists
A benzothiazole and phenyl technology, applied in the field of diamine derivatives, can solve the problems of hindering rescue treatment and maintenance treatment, and slow onset of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0401] 7-{2-[2-(3-{[2-(2,6-Dichloro-phenyl)-ethylamino]-methyl}-phenyl)-ethylamino]-1(R)- Hydroxy-ethyl}-4-hydroxy-3H-benzothiazol-2-one bis(trifluoroacetate)
[0402]
[0403] a) (3-Formyl-phenyl)-ethyl acetate
[0404] N-Bromosuccinimide (1.78 g) and 2,2'-azobis(2-methylpropionitrile) (16 mg) were added to ethyl 3-methylphenylacetate (1.76 mL) in chloroform (18 mL) solution, heated to reflux for 3 hours. After cooling the mixture was diluted with chloroform (40 mL), washed sequentially with saturated aqueous sodium bicarbonate (2 x 50 mL), brine (50 mL), dried (Na2SO4) and concentrated. The residue was dissolved in nitrogen degassed dimethyl sulfoxide (50 mL) and sodium bicarbonate (13.5 g) was added. The mixture was heated at 100 °C for 30 min under nitrogen atmosphere. The reaction mixture was cooled in an ice bath and poured into brine (300 mL). The aqueous phase was extracted with diethyl ether (3 x 300 mL). The combined organic phases were dried (sodium sulfate...
Embodiment 2
[0415] 7-[2-(2-{3-[(2,2-Diphenyl-ethylamino)-methyl]-phenyl}-ethylamino)-1(R)-hydroxy-ethyl]- 4-Hydroxy-3H-benzothiazol-2-one bis(trifluoroacetate)
[0416]
[0417] a) (3-{[tert-butoxycarbonyl-(2,2-diphenyl-ethyl)-amino]-methyl}-phenyl)-acetic acid
[0418] Prepared from (3-formyl-phenyl)-ethyl acetate (example 1, step a, 290 mg) and 2,2-diphenylethylamine (280 mg) using the method of example 1 (step b) , to give the subtitle compound as an oil (314mg).
[0419] m / z 444(M-H) - (APCI)
[0420] b) 2-{3-[(2,2-diphenyl-ethylamino)-methyl]-phenyl}-N-[2(R)-hydroxyl-2-(4-hydroxyl-2-oxo Generation-2,3-dihydro-benzothiazol-7-yl)-ethyl]-acetamide
[0421] Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (160 mg) to 7-(2-amino-1(R)-hydroxy-ethyl)-4-hydroxy -3H-Benzothiazol-2-one hydrochloride (190mg), (3-{[tert-butoxycarbonyl-(2,2-diphenyl-ethyl)-amino]-methyl}-phenyl )-acetic acid (314 mg) and 4-dimethylaminopyridine (210 mg) in dimethylformamide (10 mL) and st...
Embodiment 3
[0429] 7-[2-(2-{3-[(2-Chloro-benzylamino)-methyl]-phenyl}-ethylamino)-1(R)-hydroxy-ethyl]-4-hydroxy- 3H-Benzothiazol-2-one bis(trifluoroacetate)
[0430]
[0431] a) (2-chlorobenzyl)-[3-(2-hydroxy-ethyl)-benzyl]-tert-butyl carbamate
[0432] 2-Chlorobenzylamine (0.28 g) was added to a solution of ethyl (3-formyl-phenyl)-acetate (Example 1, step a) (0.38 g) and acetic acid (114 μL) in ethanol (10 mL). After 1 hour, sodium triacetoxyborohydride (1.27 g) was added and the reaction mixture was stirred for 18 hours. After adding a few drops of 0.880 aqueous ammonia, the reaction mixture was concentrated. The residue was dissolved in ethanol (5 mL) and loaded onto a conditioned SCX cartridge (10 g Varian). The cartridge was washed with ethanol (3 x 20 mL) and eluted with ethanol / 0.880 ammonia solution [4:1] (2 x 15 mL). The combined eluted fractions were evaporated. The residue was dissolved in dimethylformamide (5 mL), and a solution of di-tert-butyl dicarbonate (0.48 g) in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com