Freeze-dried powder preparation of kuh-seng native, preparation method thereof
A technology of matrine and freeze-dried powder, which is used in freeze-drying transportation, powder transportation, antiviral agent and other directions, can solve the problems of unsatisfactory resolution, affecting the accuracy of content determination, and poor peak shape of spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
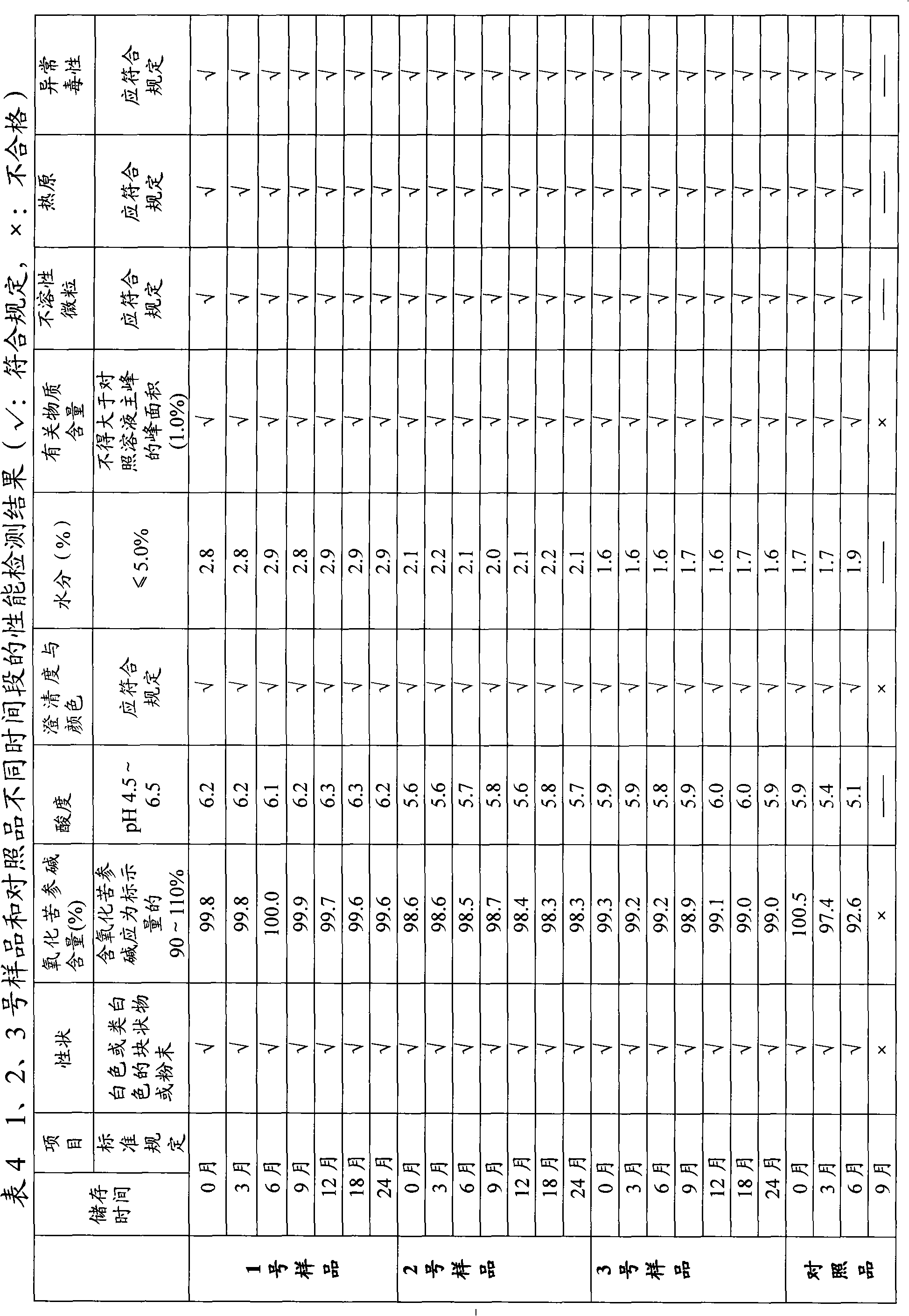
[0010] Weigh 100 g of Sophora flavescens coarse powder, soak it with 10 times the amount of 60% ethanol solution for 24 hours, and then carry out percolation at a flow rate of 5 ml / min. After the percolation liquid is concentrated under reduced pressure, it is dissolved with an appropriate amount of purified water, filtered, applied to AB-8 macroporous adsorption resin, and eluted with 70% ethanol solution. After recovering ethanol from the eluent, add hydrogen peroxide for oxidation until the oxidation is complete, and then add a reducing agent Reduce excess hydrogen peroxide, evaporate the solution to dryness, crystallize with acetone, and recrystallize to obtain white matrine crystals.
[0011] After testing, matrine contains oxymatrine (C 15 h 24 N 2 o 2 )99.6%.
Embodiment 2
[0013] Prescription Composition Screening Test
[0014] In view of the poor stability of the existing matrine freeze-dried powder preparations, it is considered to add antioxidants. Sodium bisulfite, edetate disodium and L-cysteine hydrochloride were respectively selected for testing. Results The addition of sodium bisulfite and L-cysteine hydrochloride affected the inspection of related substances in the preparation, and disodium edetate could improve the color of the preparation solution without interfering with the inspection of related substances, so it could be applied to this preparation. At the same time, a series of varieties of edetate calcium sodium edetate disodium were tested, and the results showed that edetate calcium sodium sodium was also applicable.
[0015] Further screen the amount of antioxidants. The amount of matrine and mannitol is fixed, the dosage of edetate disodium or edetate calcium sodium is 0.05%, 0.1%, 0.2%, 0.3%, 0.5%, 0.6% of the dosage o...
Embodiment 3
[0017] Preparation of matrine freeze-dried powder preparation
[0018] Weigh mannitol, add 40% of the prepared amount of water for injection, heat and stir until completely dissolved, add medicinal charcoal and boil for 15 minutes, set the volume to 50% of the prepared amount, cool to 80°C and filter to remove charcoal. Add matrine to 30% of the prepared amount of water for injection, stir to dissolve, add 2mol / L hydrochloric acid to adjust the pH value, add edetate disodium or edetate calcium sodium solution, add water for injection to 40% of the prepared amount , heated to 70-80°C, added medicinal charcoal, stirred and adsorbed for 15 minutes, and filtered to remove charcoal. Cool the mannitol solution after carbon removal to below 50°C, combine it with the matrine solution after carbon removal, add water to the full amount, stir evenly, and adjust the pH value to 4.8-6.5 with 2mol / L hydrochloric acid. After the temperature of the medicinal solution drops to room temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com