Comestible compositions comprising high potency savory flavorants, and processes for producing them
A composition and compound technology, applied in the preparation of food and beverages, the preparation of some umami tastant compounds disclosed in this paper, and the preparation of food seasoning concentrate compositions, which can solve the problem that the compound is not optimal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
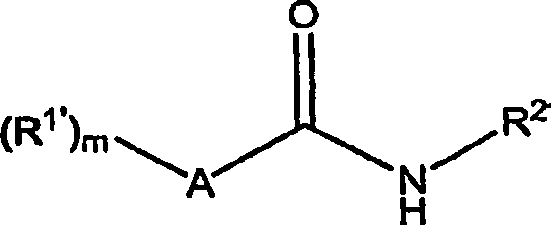
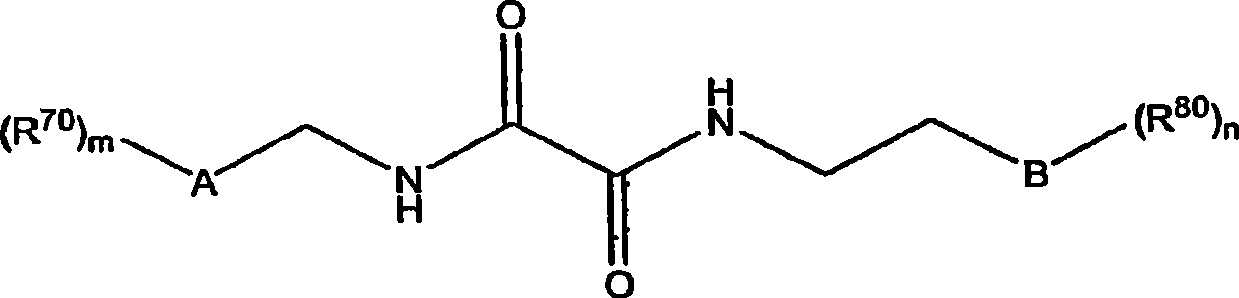
[0320] The preparation of formula (I) amide compound
[0321] The starting materials for the preparation of various structural subclasses and specific substances of the compounds of the present invention, amide compounds of formula (I) and their synthetic precursors, and methods for preparing the above compounds are disclosed in U.S. Patent Publication Nos. US 2005 / 0084506 A1 and These two patent applications are incorporated herein by reference in US Patent Publication No. US 2006 / 0045953 Al, or as described below.
[0322] resolve resolution
[0323] The following schemes and examples are provided to guide the reader and represent various methods for preparing the amide compounds of this invention. The methods disclosed are for purposes of illustration only and are not limiting, and it will be apparent to those skilled in the art that other methods, many of which are well known, may be used to prepare the amide compounds in various embodiments of the invention. These met...
Embodiment 1
[0500] N-(hept-4-yl)benzo[d][1,3]dioxol-5-carboxamide
[0501]
[0502] To a solution of hept-4-amine (8.06 mL, 54 mmol) in triethylamine (15.3 mL, 108 mmol) and dichloromethane (135 mL) was added dropwise benzo[1,3]dioxole at 0 °C -5-Acyl chloride (10 g, 54 mmol) in dichloromethane (135 mL). The reaction mixture was stirred for 1 h. The solvent was removed under reduced pressure and the residue was dissolved in EtOAc (ethyl acetate). The organic layer was washed successively with 1N aqueous HCl, 1N aqueous NaOH, water, brine, dried (MgSO4), and concentrated. The residue was recrystallized from EtOAc and hexanes to afford 6.9 g of N-(hept-4-yl)benzo[d][1,3]dioxole-5-carboxamide (48.3% ). 1 H NMR (500MHz, CDCl 3 ): δ0.92(t, 6H), 1.38(m, 6H), 1.53(m, 2H), 4.11(m, 1H), 5.63(m, 1H), 6.01(s, 2H), 7.98(d, 1H), 7.27 (s, d, 2H). MS (M+H, 264).
[0503] This compound activates the EC of hT1R1 / hT1R3 umami taste receptors expressed on HEK293 cell line 50 It is 0.2μM, and when...
Embodiment 1-1
[0505] Improvements in the preparation and purification of N-(hept-4-yl)benzo[d][1,3]dioxole-5-carboxamide:
[0506]
[0507] In a clean fume hood, place a 3-neck round bottom flask equipped with a mechanical stirring assembly, addition funnel, thermocouple with display, nitrogen gas inlet, and drying tube in a cooling bath. The flask was also blanketed with nitrogen. 674 g of 4-heptylamine (1 equivalent, 5.85 moles) was added to the flask under nitrogen protection. THF (tetrahydrofuran) (3.37 L) was then added to the flask and the reaction mixture was stirred. Then triethylamine (1184 g, 2 eq, 11.7 mol) was added to the reaction mixture under nitrogen protection, and the reaction mixture was cooled to an internal temperature of -5°C to 0°C.
[0508] Piperonyl chloride (1080 g, 5.85 mol) was dissolved in THF (3.37 L) in a polyethylene jar and stored under nitrogen. Transfer this solution to the addition funnel of the 3-neck flask. This solution was added portionwise to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com