Preparation of poplar antibacterial compounds and application thereof as bactericidal agent
A compound, poplar technology, applied in the field of pesticides, can solve the problems of tracking and separating antibacterial compounds, which have not yet been seen, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Small-scale preparation of different polarity extracts of poplar
[0020] Take European and American 107 poplar branches, dry them indoors, grind them, take 900g, and use 95% ethanol (sample: ethanol = 1:5, g / mL) to reflux extract 3 times at 80°C, each time for 1h, and combine 3 The sub-ethanol extract was concentrated under reduced pressure to obtain a crude ethanol extract (60.81 g, yield 6.76%). Take 52.93 g of the ethanol crude extract, heat and dissolve with distilled water to make a suspension, extract with ethyl acetate and n-butanol in sequence, concentrate the ethyl acetate layer, n-butanol layer, and the last remaining water layer under reduced pressure to obtain Ethyl acetate fraction (32.95g, 4.21% yield), n-butanol fraction (10.53g, 1.34% yield) and water fraction (8.21g, 1.05% yield).
Embodiment 2
[0021] Example 2: Tracking separation and preparation of poplar antibacterial compounds
[0022] Take European and American 107 poplar branches, dry them indoors, crush them, take 5.79kg, and use 95% ethanol (sample: ethanol = 1:5, g / mL) to reflux extract at 80°C for 3 times, each time for 1h, and combine 3 times The ethanol extract was concentrated under reduced pressure to obtain a crude ethanol extract (430.16 g). Take 420.00 g of the ethanol crude extract, heat and dissolve it with distilled water to make a suspension, extract it with ethyl acetate and n-butanol successively, concentrate the ethyl acetate layer, n-butanol layer, and the last remaining water layer under reduced pressure to obtain Ethyl acetate fraction (236.32g), n-butanol fraction (135.70g) and water fraction (46.23g). For the ethyl acetate extraction part with better antibacterial activity, the active components were further tracked and separated, and normal pressure normal phase or reverse phase silica ...
Embodiment 3
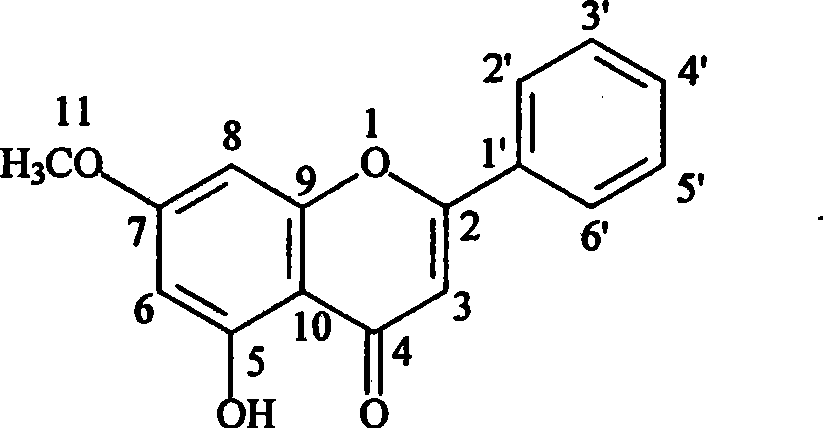
[0023] Embodiment 3: Structural identification of fatty alkanol p-hydroxycinnamate (1)
[0024] A white powder (chloroform) was prepared according to the method described in Example 2. After developing a normal-phase silica gel plate with petroleum ether: acetone (2:1, v / v) system, a single dark spot was visible under 254nm ultraviolet light. (Rf=0.70), the sulfuric acid chromogen showed dark yellow spots.
[0025] 1 H NMR (CDCl 3 , 300MHz), aromatic region δ: 7.63ppm (1H, d, J = 15.96Hz) and 6.30ppm (1H, d, J = 15.96Hz) signals indicate that there is a trans double bond in the molecule; δ: 7.43ppm ( 2H, m) and 6.83ppm (2H, m) indicate that there is a para-substituted benzene ring in the molecule; the broad single peak at δ: 5.22ppm (1H, brs) indicates that there is a hydroxyl substitution in the benzene ring, 13 C NMR (CDCl 3 , 75MHz), δ: The signal of 157.5ppm also verified that there is a hydroxyl substitution in the benzene ring; 1 The proton signal in the H NMR high-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com