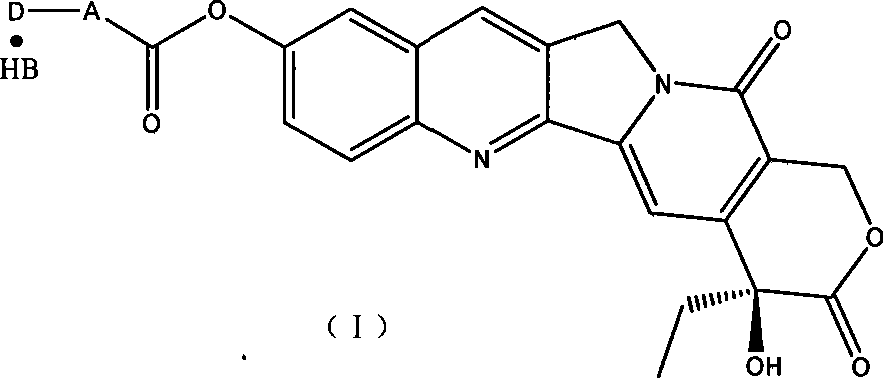
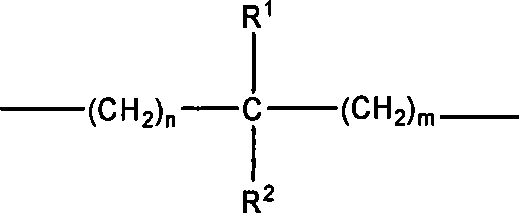
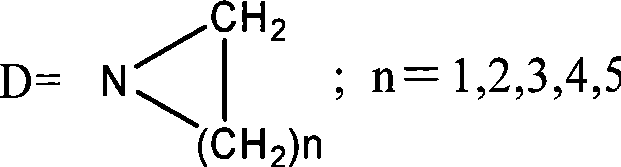Water-soluble derivates containing 10-hydroxycamptothecin and preparation method
A technology of hydroxycamptothecin and its derivatives, applied in the field of water-soluble derivatives and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Hydroxycamptothecin 10-(γ-N,N-Diethylamino)butyrate Hydrochloride
[0032] Under a dry nitrogen flow, add 80 mg of 10-hydroxycamptothecin and 4 ml of anhydrous pyridine into a 25 ml dry three-necked flask, and stir at room temperature until completely dissolved. Dissolve 300 mg (γ-N, N-diethylamino) butyryl chloride hydrochloride in 5 ml of dry dichloromethane, slowly add it dropwise, and react at room temperature for 2-3 hours. After the reaction is complete, add a few drops of water to the reaction solution, stir for 5 minutes, add 15ml of ether, and precipitate a solid, filter it, and rinse the solid with ether. Dissolve the solid in methanol, add diethyl ether dropwise until it becomes turbid, static crystallize, suction filter, rinse with diethyl ether; recrystallize according to the above method, dry under reduced pressure to obtain 60 mg of light yellow solid, m.p221-223°C, harvested rate 54%. IR (KBr, cm -1 ): 3559, 3435, 2942, 2646, 1774, 1743...
Embodiment 2
[0035] Preparation of Hydroxycamptothecin 10-(γ-Amino)butyrate Acetate
[0036] Dissolve 280 mg of CBZ-protected GABA and 250 mg of DCC in 10 ml of CH 2 Cl 2 In, add 40mg DMAP, stir at room temperature for 10min, then add 0.5ml pyridine and 150mg HCPT. After reacting for more than 10 hours, filter, and wash the filtrate with pH3 acid water until the water layer is acidic. NaHCO 3 Wash until the aqueous layer is alkaline, and finally wash with saturated brine until neutral. The organic layer was washed with anhydrous Na 2 SO 4 Dry and evaporate the organic solvent. Dissolve in THF, add 0.5ml of acetic acid, a little 10% palladium-carbon, pass through hydrogen for hydrogenolysis, react for about 1 hour, filter, add ether to crystallize, and obtain 100mg of light yellow solid, yield 46%. IR (KBr, cm -1 ): 3428, 2927, 1745, 1657, 1623, 1591, 1504, 1235, 1193, 1162, 1109, 1047. ESI-MS: 450 (M+ -AcOH)
Embodiment 3
[0038] Preparation of HCPT-γ—aminobutyrate hydrochloride
[0039] Dissolve 230mg of Boc-protected γ-aminobutyric acid and 250mg of DCC in 10ml of CH 2 Cl 2 In, add 40mg DMAP, stir 10min. Add 0.5ml of pyridine and 150mg of HCPT, and react for more than 10 hours. After the reaction is completed, wash with pH3 acidic water until the water layer is acidic, then wash with saturated NaHCO3 until the water layer is alkaline, and finally wash with saturated saline until the water layer To be neutral, the organic layer was dried with anhydrous NaSO4. The organic solvent was evaporated to obtain 200 mg of a light yellow solid with a yield of 85%. Dissolve the solid in a mixture of ethyl acetate and dichloromethane, add HCl-saturated ethyl acetate solution dropwise under ice-bath cooling, filter after the reaction is complete, dissolve the filter cake in water, add acetone, crystallize, and filter 20 mg of HCPT-γ-aminobutyrate hydrochloride was obtained. Yield 11%. ESI-MS: 450 (M ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com