Dipeptidase-IV inhibitor derivates
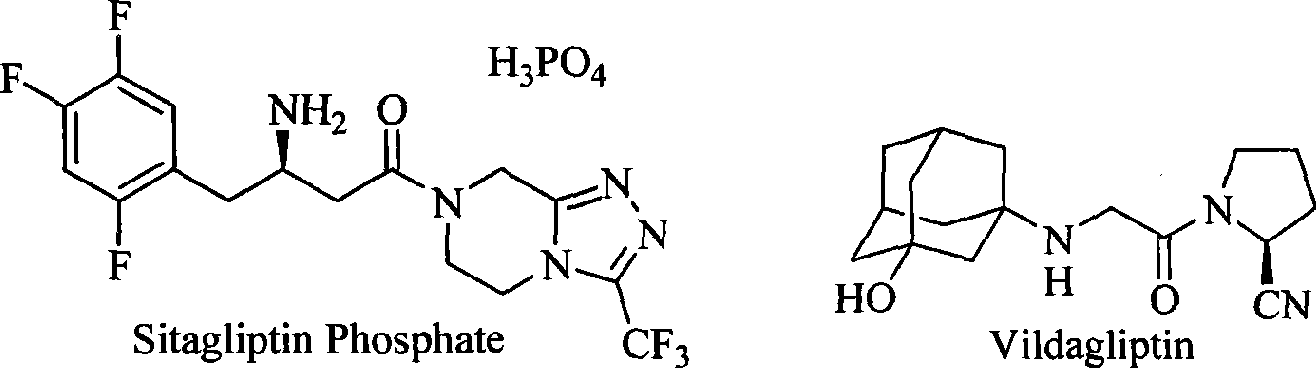
A technology for drugs and compounds, applied in the field of medicine, can solve the problems of inability to meet clinical needs, limited varieties of DPP-IV inhibitors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
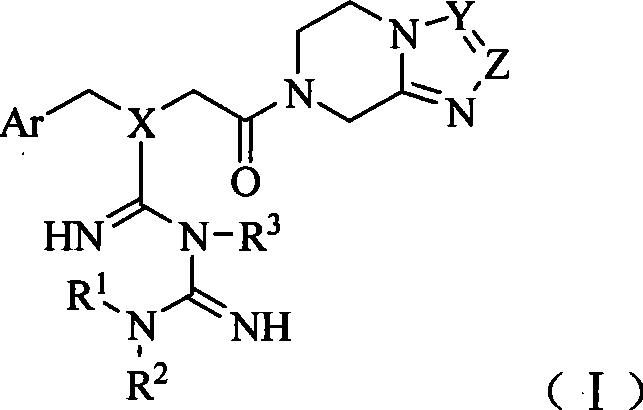
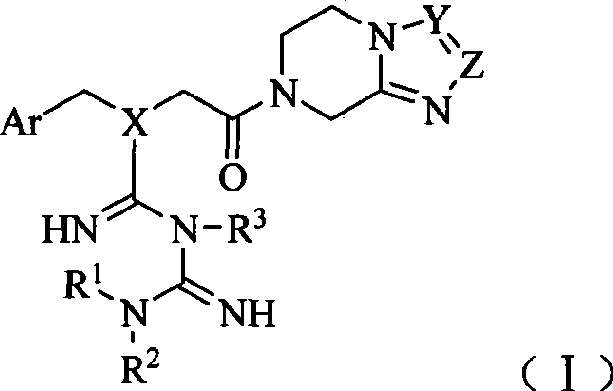
[0134] Example 1 7-[2-[N-(2,4,5-trifluorobenzyl)-N-((1,1-Metguanidine-3-yl)iminomethyl)aminoacetyl ]-3-(three Preparation of fluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazol[4,3-a]pyrazine
[0135] Step 1 7-(2-Chloro-acetyl)-3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazol[4,3-a]pyridine Preparation of oxazine
[0136] In a dry reaction flask, add 9.6g (50mmol) 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a] Pyrazine (see J.Med.Chem.2005, 48, 141-151 for its preparation method), 100ml of DMF, after slightly heating and stirring to dissolve, lower to -5~0°C, slowly add 6.8g (60mmol) of chloroacetyl chloride dropwise Dichloromethane solution 100ml, dropwise, keep warm for 5h. Then warmed to room temperature and stirred for 0.5h. 100ml of water was added to the reaction solution, and the pH was slowly adjusted to 9 with 5% sodium bicarbonate solution under an ice-water bath. The organic layer was separated, and the aqueous layer was extracted with 50ml×...
Embodiment 2
[0146] Embodiment 2 The preparation of compound tablet of the present invention
[0147] 1. Prescription:
[0148]
[0149] 2. Preparation process: crush the raw materials through a 100-mesh sieve, pass the rest of the auxiliary materials through a 100-mesh sieve, and set aside; weigh the raw materials and auxiliary materials according to the prescription; mix compound 1, microcrystalline cellulose and hydroxypropyl cellulose evenly, add Add an appropriate amount of water to the mixing granulator, stir and granulate; dry the wet granules at a temperature lower than 60°C; add micropowder silica gel and magnesium stearate to the dried granules; take samples and test semi-finished products; The determined tablet weight is pressed; the finished product is fully inspected, packed and put into storage.
Embodiment 3
[0150] Embodiment 3 Preparation of compound capsules of the present invention
[0151] 1. Prescription:
[0152]
[0153] 2. Preparation process: crush the raw materials through a 100-mesh sieve, pass the rest of the auxiliary materials through a 100-mesh sieve respectively, and set aside; weigh the raw materials and auxiliary materials according to the prescription; mix compound 1, microcrystalline cellulose and starch evenly, and add them to the mixing granulator Add appropriate amount of water, stir and granulate; dry the wet granules at a temperature lower than 60°C; add micro-powder silica gel and magnesium stearate to the dried granules; take samples and test the semi-finished products; determine the loading according to the test; Capsule loading, full inspection of finished products, packaging and storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com