Pseudomonas putida formaldehyde dehydrogenase gene, encoded protein and recombinant vector
A technology of Pseudomonas putida and formaldehyde dehydrogenase, applied in genetic engineering, plant gene improvement, recombinant DNA technology, etc., can solve the problems of high price and achieve high application prospects and high industrial development value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Pseudomonas putida PFDH gene amplification
[0028]Primer design: According to the gene terminal sequences of several GSH-independent formaldehyde dehydrogenases in Pseudomonas putida reported in NCBI, two primers were designed and synthesized (the 5' end contains NdeI and XhoI restriction sites, respectively).
[0029] Sense strand primer: 5'GGAGTTCCATATGTCTGGCAATCGTGGAGTGGT3'
[0030] Antisense strand primer: 5'CCTACTCGAGCGCCGCACCCCACATCTTGTGCG3'
[0031] Colony PCR amplification: A single colony of Pseudomonas putida AS 1.643 was added to 15 μl of ultrapure water, heated at 100° C. for 5 minutes, and centrifuged at 13,000 rpm for 1 minute to obtain a lysate. For each 50 μl PCR reaction volume, add 10 μl of the above lysate as a template, 1 μl each of 25 μM sense strand primer and antisense strand primer, 1 μl 10 mM dNTP, 5 μl 10×Taq plus DNA polymerase buffer, 5 μl 100ml / LDMSO and 26.2 μl ultrapure water . Put the thin-walled tube containing the above re...
Embodiment 2
[0032] Example 2 Construction of recombinant Pseudomonas putida PFDH expression plasmid
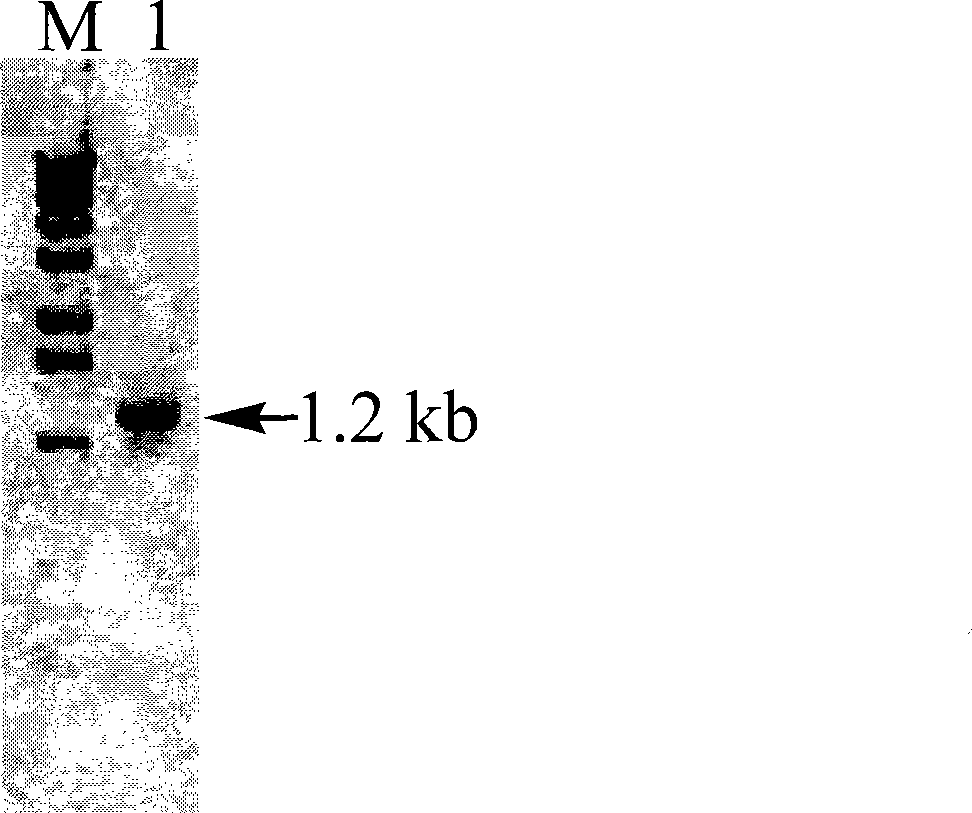
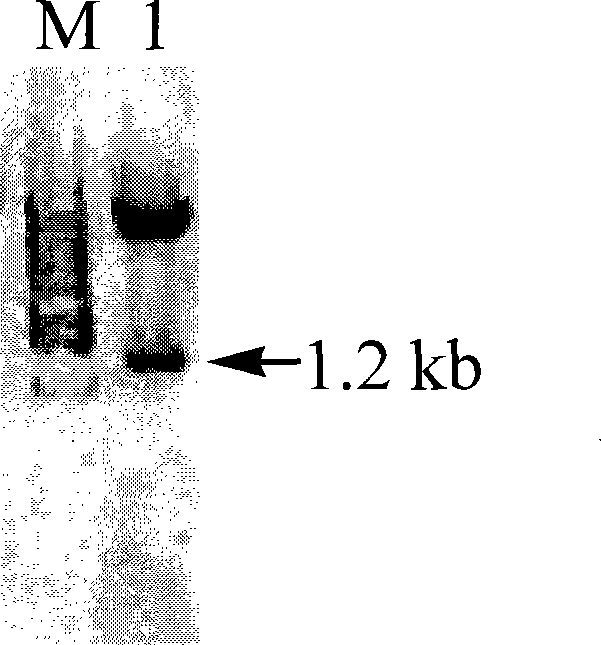
[0033] The above PCR product was recovered, digested with NdeI / XhoI, and cloned into the prokaryotic expression vector pET24b(+) ( figure 2 ). Transform E.coli DH5α, and culture the transformant in LB medium containing kanamycin (30 μg / ml), extract the plasmid, and carry out double enzyme digestion identification with NdeI / XhoI, and a band of about 1.2 kb can be obtained ( image 3 ), proving that the recombinant plasmid has been successfully inserted into the PFDH gene fragment.
[0034] The foreign gene in the recombinant expression vector is determined by DNA sequence, and the identified length is 1197bp, and the encoded protein contains 399 amino acid residues. Sequence comparative analysis shows that the nucleotide sequence homology of this gene and the reported (ItoK.etal.J.Bacteriol.176:2483-2491, 1994) Pseudomonas putida PFDH is 85.3%, and has a typical The characteristics of ...
Embodiment 3
[0035] Example 3 Expression of recombinant PFDH
[0036] Transform pET24b-PFDH into E.coli BL21(DE3) to obtain genetically engineered bacteria. The engineered bacteria were induced and cultivated in LB medium containing kanamycin (30 μg / ml), the bacteria were collected, lysed by ultrasonic waves, and the supernatant was analyzed by SDS-PAGE, which showed that obvious specific expression product bands were obtained, and the molecular weight Consistent with the theoretical value of PFDH (43kDa) predicted by software DNAStar ( Figure 4 ), indicating that recombinant PFDH was successfully induced to express.
[0037] The culture conditions for recombinant PFDH induced expression were optimized and determined as follows: Inoculate a single colony in 70ml LB medium containing 30μg / ml kanamycin, culture overnight at 37°C, 200rpm, take 64ml of the overnight culture and inoculate it in 3.2L containing 30μg / ml kanamycin ml kanamycin LB medium, 37°C, 200rpm culture to OD 600 =0.64 (a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com