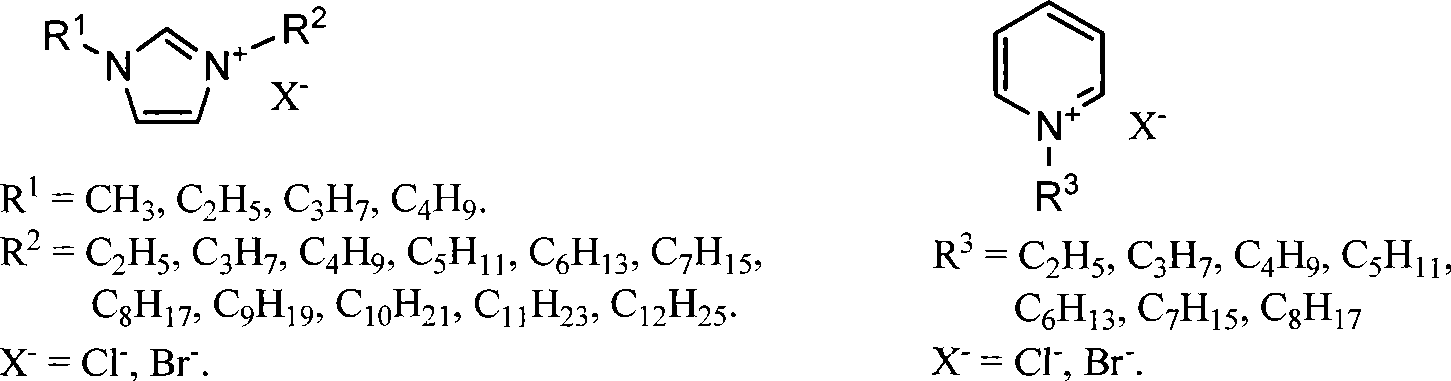Method for preparing 5-hydroxymethyl-furfural
A technology of methyl and substituents, which is applied in the field of preparation of 5-hydroxymethylfurfural, can solve the problems that the research situation has not been reported, the ionic liquid is not recycled, and the principle of green chemistry is hindered, and the separation method of the product is simple. Ease of operation, less catalyst and solvent consumption, less demanding effects on corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 4 g of ionic liquid [C 4 MIm]Cl was added to a 10 ml round bottom flask, heated to 80°C, and 0.4 g of fructose was slowly added under vigorous stirring, and stirred until dissolved. At this point, 0.02 g of concentrated hydrochloric acid was quickly added to the reaction system, and reacted at 80° C. for 8 minutes under normal pressure to stop the reaction. Get 0.01 gram sample and quench reaction immediately with cold water, this sample pH value is adjusted to 7.0 with the NaOH of 0.05mol / L, gained aqueous solution measures HMF productive rate at 282nm place with ultraviolet-visible spectrophotometer by standard curve method and is 97%.
[0039] The remaining part in the reaction system was separated by column chromatography (using silica gel as filler, eluent: petroleum ether / ethyl acetate=4:2 (v / v)), to obtain 0.269 g of dark yellow liquid product HMF, The isolated yield was 96%. The product is analyzed by nuclear magnetic resonance spectroscopy, and the data are: ...
Embodiment 2
[0041] 4 g of ionic liquid [C 4 MIm]Cl was added to a 10 ml round bottom flask, heated to 80°C, and 4 g of fructose was slowly added under vigorous stirring, and stirred until dissolved. At this point, 0.2 g of concentrated hydrochloric acid was quickly added to the reaction system, and reacted at 80° C. for 25 minutes under normal pressure. Reaction finishes, quenches reaction with cold water, with the NaOH of 0.05mol / L the pH value of reaction solution is adjusted to 7.0, gained aqueous solution measures HMF productive rate at 282nm place by standard curve method and is 77% with ultraviolet-visible spectrophotometer, isolates product The rate is 75%.
Embodiment 3
[0043] 4 g of ionic liquid [C 4 MIm]Cl was added to a 10 ml round bottom flask, heated to 80°C, and 8 g of fructose was slowly added under vigorous stirring, and stirred until dissolved. At this point, 0.4 g of concentrated hydrochloric acid was quickly added to the reaction system, and reacted at 80° C. for 120 minutes under normal pressure. Reaction finishes, quenches reaction with cold water, with the NaOH of 0.05mol / L the pH value of reaction solution is adjusted to 7.0, gained aqueous solution measures HMF productive rate at 282nm place by standard curve method and is 58% with ultraviolet-visible spectrophotometer, isolates product The rate is 55%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com