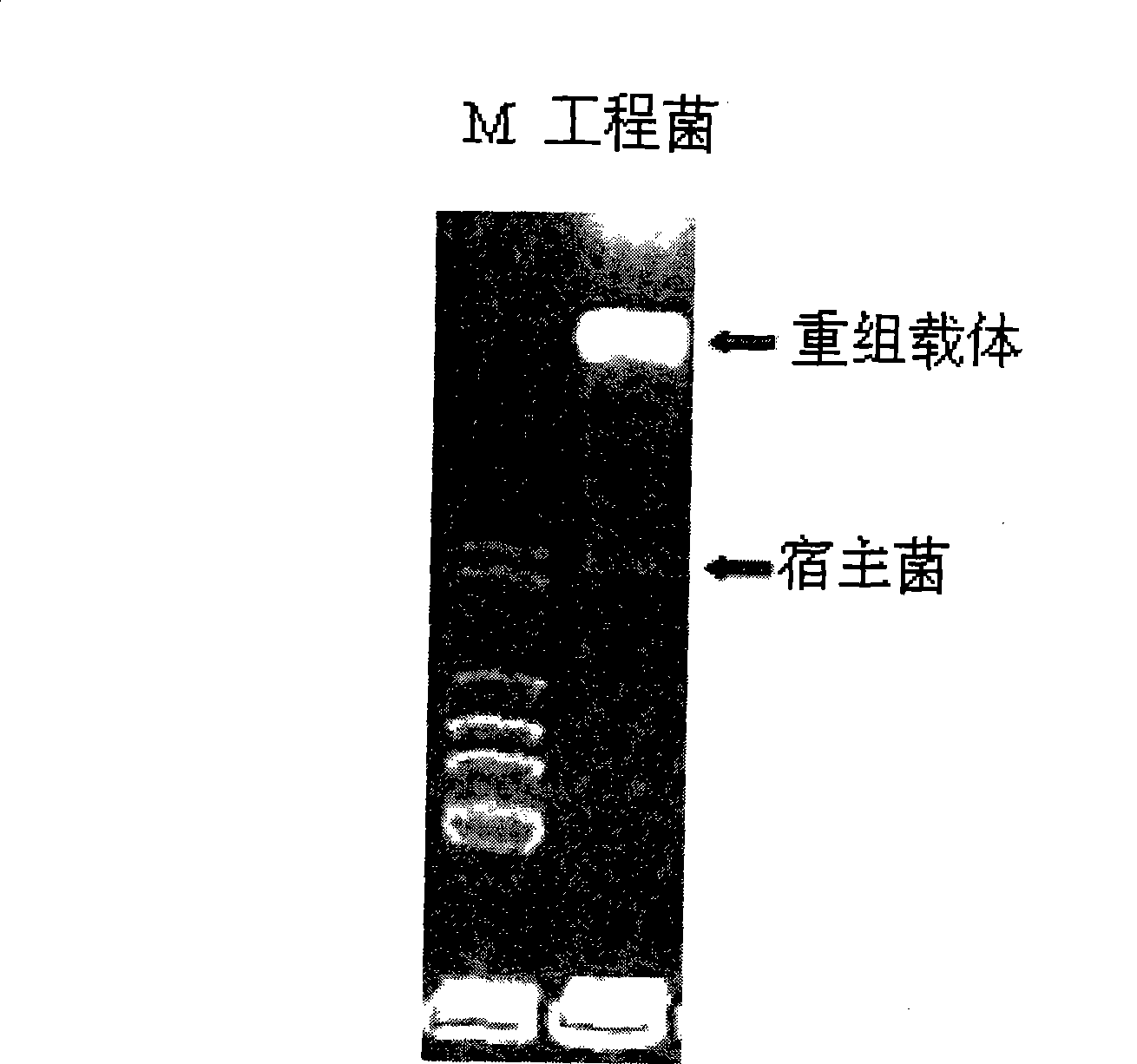
Construction of human insulinogen C peptide high-yield strains
A technology of human insulin and high-efficiency cells, which is applied in the direction of fungi, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of increasing separation and purification steps, reducing the yield of human proinsulin C-peptide, and achieving low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Example 1 The construction process of the recombinant expression vector containing human proinsulin C peptide
[0039] Chemical synthesis of human proinsulin C-peptide gene: According to the amino acid sequence of human proinsulin C-peptide (SEQ ID NO: 2), two complementary nucleotide sequences were synthesized.
[0040] The forward nucleotide sequence contains the following sequence (5'→3'): gaggccgaagatta cag gtaggacaagttgagttgggaggaggcccaggggcaggctcgctacagcccttggccttggaaggctcactgcag;
[0041]The reverse nucleotide sequence contains the following sequence (5'→3'):
[0042] ctgcagtgagccttccaaggccaagggctgtagcgagcctgcccctggggcctcctcccaactcaacttgtcctacctgtaaatcttcggcctc.
[0043] Restriction endonuclease EcoR I was designed at the 5' end of the forward and reverse nucleotide sequences.
[0044] The human proinsulin C-peptide gene is obtained by annealing the two complementary nucleotide sequences. The above-mentioned nucleic acid product and expression vector pPIC3K we...
example 2
[0047] Example 2 Shake flask expression of human proinsulin C-peptide situation
[0048] Pick a single colony from the solid plate, inoculate into a 250mL shake flask containing 25mL BMGY, grow overnight at 30°C, 220rpm to OD 600 =4.
[0049] Inoculate the above 25mL culture solution into 1LBMGY, and continue to amplify to the cell concentration OD 600 =6.
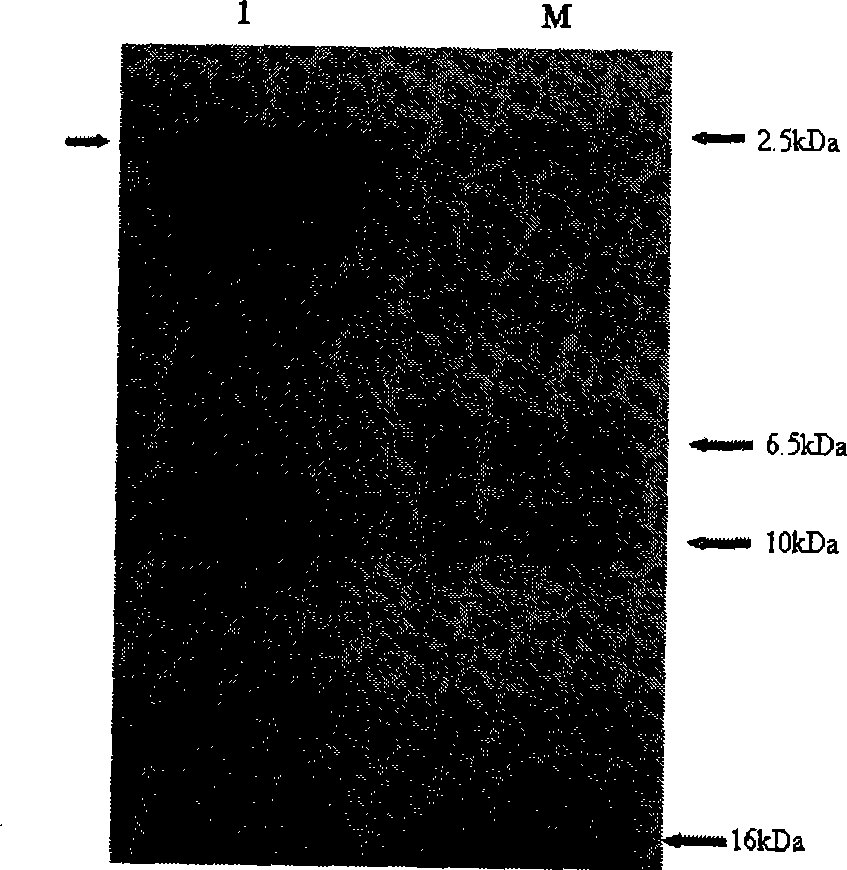
[0050] Centrifuge at 5000rpm for 5min at room temperature to harvest the cells. Suspend the bacteria with fresh sterile BMMY to the OD of the bacteria solution 600 =1.0, 30°C, 220rpm induced expression of human proinsulin C-peptide. Samples were taken every 24 hours and supplemented with 100% methanol to a final concentration of 1.5%. After 120 hours of induction culture, the cells were harvested by centrifugation. The bacteria were analyzed by protein electrophoresis, and the results were as follows: figure 2 As shown, the results show that: human proinsulin C-peptide was expressed in Pichia pastoris.
[0051] Sh...
example 3
[0052] Example 3 Fermentative expression of human proinsulin C-peptide
[0053] Pick a single colony from the solid plate, inoculate into a 250mL shake flask containing 25mL BMGY, grow overnight at 30°C, 220rpm to OD 600 =4.
[0054] Inoculate the above 25mL culture solution into 250mL BMGY, and continue to amplify to the cell concentration OD 600 =6, inoculate the culture solution into 2L BSM fermentation medium, adjust pH=4.0-7.5, temperature at 28°C, DO=40%, rotating speed 500rpm, after cultivating for a period of time, DO rises rapidly to more than 100%, indicating After the consumption of glycerol in the medium was completed, 50% glycerol was added slowly, the glycerol flow rate was 30mL / h, and the DO was maintained above 35%, and the glycerol supplementation time was 4h.
[0055] Methanol induction stage: After stopping glycerol supplementation, DO rises rapidly to above 100%. After starvation for a period of time, methanol is slowly supplemented. The methanol flow rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com