Modified recombinant human endostatin and use thereof
A technology of endostatin and weight-average molecular weight, which is applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of difficult purification of biologically active mixtures, and achieve anti-endothelial cell proliferation Effects of increased activity, reduced economic burden, and reduced dosing frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, CH with a weight average molecular weight of 20kd 3 O-(CH 2 CH 2 O) 20kD -CH 2 CH 2 CH(OCH 2 CH 3 ) 2 Conjugates with Endostar (abbreviated as PEG 20 -ENDO) preparation:
[0040] (1)CH 3 O-(CH 2 CH 2 O) 20kD -CH 2 CH 2 CH(OCH 2 CH 3 ) 2 Conjugation to Endostar
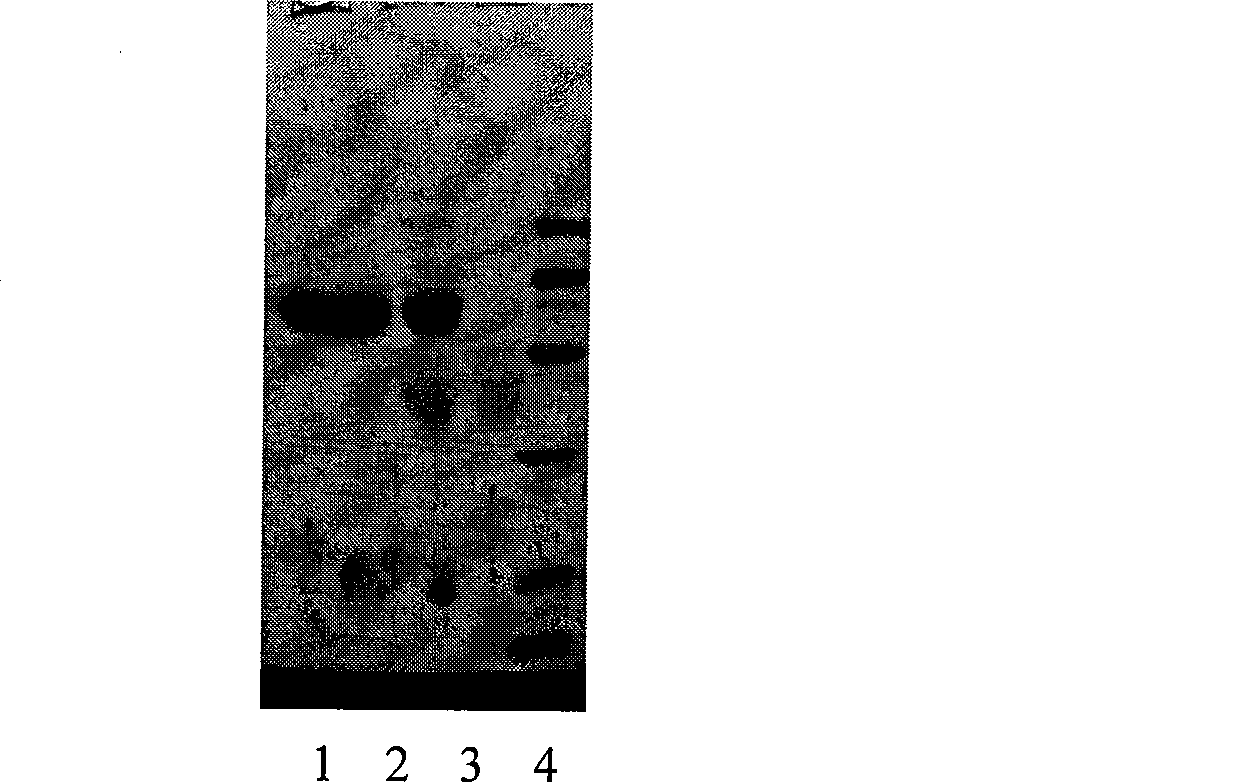
[0041] 20ml Endostar stock solution (PH5.0~5.5, HAC / NaAC Buffer, protein content 5mg / ml), add reducing agent sodium cyanoborohydride NaCNBH 3 To a final concentration of 20mM, add 100mg of monomethoxypolyethylene glycol-3,3-diethoxypropane to make the molar ratio of Endostar to 1:1, and stir overnight at 4°C to form PEG 20 -ENDO modification, the product was identified by SDS-PAGE, see the identification results figure 1 .
[0042] (2)PEG 20 -Purification of ENDO
[0043] The above reaction products were separated and purified with CM-Sepharose column and AKTA chromatography. The sample was diluted with 20mM, pH 7.0 PB buffer and loaded, equilibrated to the baseline with 20m...
Embodiment 2
[0044] Embodiment 2, weight average molecular weight is the CH of 40kd 3 O-(CH 2 CH 2 O) 40kD -CH 2 CH 2 Conjugates of CHO and Endostar (abbreviated as PEG 40 -ENDO) preparation:
[0045] (1)CH 3 O-(CH 2 CH 2 O) 40kD -CH 2 CH 2 Coupling of CHO and Endostar 20ml Endostar stock solution (PH5.0~5.5, HAC / NaAC Buffer, protein content 5mg / ml), add reducing agent sodium cyanoborohydride NaCNBH 3 To a final concentration of 20mM, add 400mg of monomethoxypolyethylene glycol-3,3-diethoxypropane to make the molar ratio of Endostar to 1:2, and stir overnight at 4°C to form PEG 40 -ENDO modification, the product was identified by SDS-PAGE. ( Figure 4 )
[0046] (2)PEG 40 -Purification of ENDO
[0047] The above reaction products were separated and purified with CM-Sepharose column and AKTA chromatography. The sample was diluted with 20mM, pH 7.0 PB buffer and then loaded, equilibrated to the baseline with 20mM, pH 7.0 PB buffer, and then gradient with 20mM, pH 7.0 PB buf...
Embodiment 3
[0048] Example 3, Pharmacokinetic study of polyethylene glycol-modified recombinant human endostatin
[0049]Fifteen male SD rats were randomly divided into 3 groups and administered at 4.5 mg / kg body weight. Group A is unmodified Endostar, group B is PEG 20 -ENDO, Group C is PEG 40 -ENDO. Blood was collected at 5min, 10min, 15min, 30min, 1h, 4h, 6h, 12h, 24h, 48h, and 96h after administration, anticoagulated with heparin, and immediately centrifuged at 6000r / min for 10min to obtain plasma samples. Using Human Endostatin Protein The EIA kit was used to determine the content of samples in plasma. The effective blood drug concentration of endostatin after the determination is modified is increased by more than 20 times ( Figure 7 ), the half-life of Endostar is 8 hours, PEG 20 -ENDO has an in vivo half-life of 18h, PEG 40 -ENDO has an in vivo half-life of 97h. Compared with Endostar, PEG 20 -ENDO's AUC increased by at least 10 times, PEG 40 -ENDO's AUC increased by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com