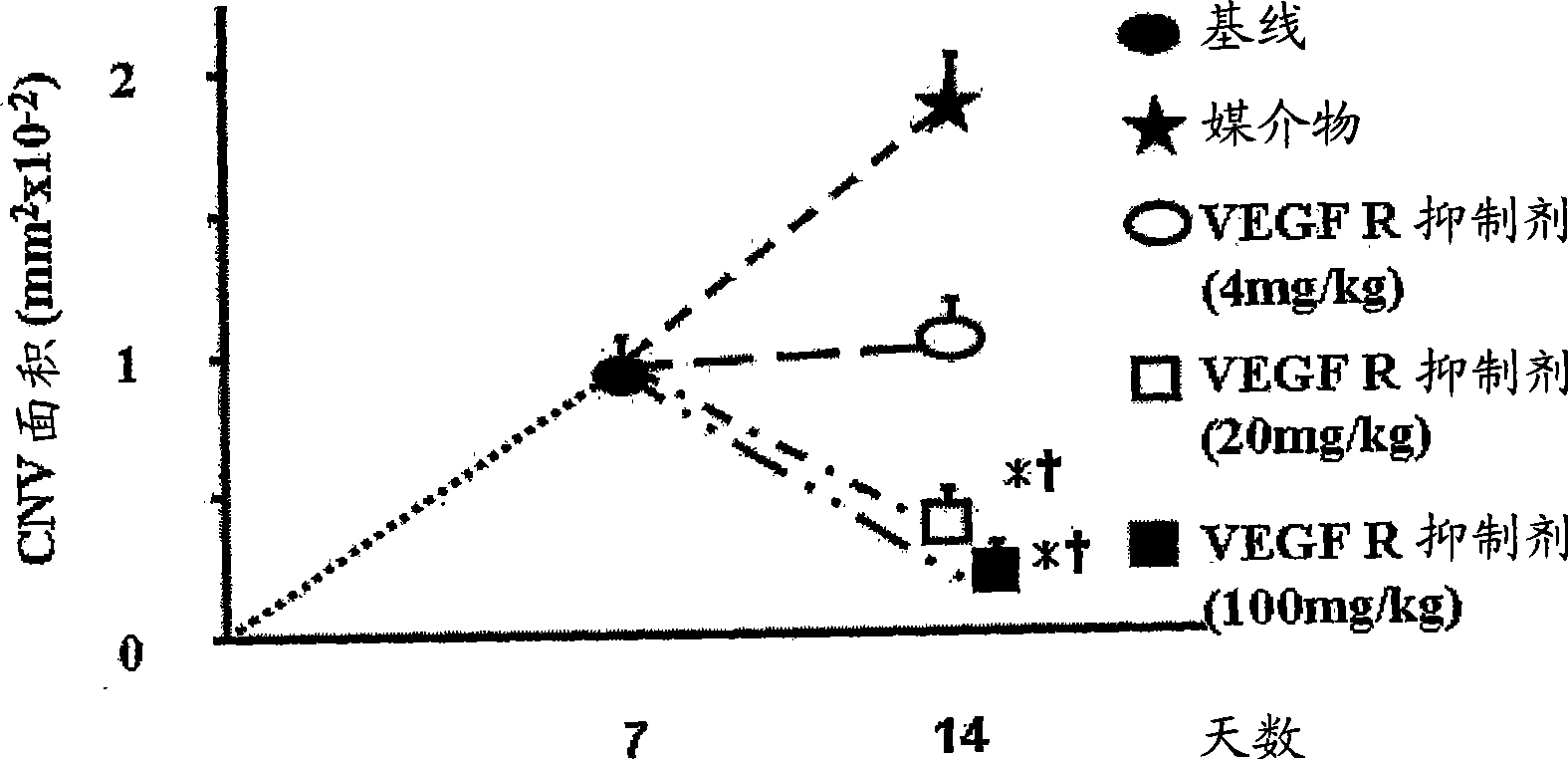
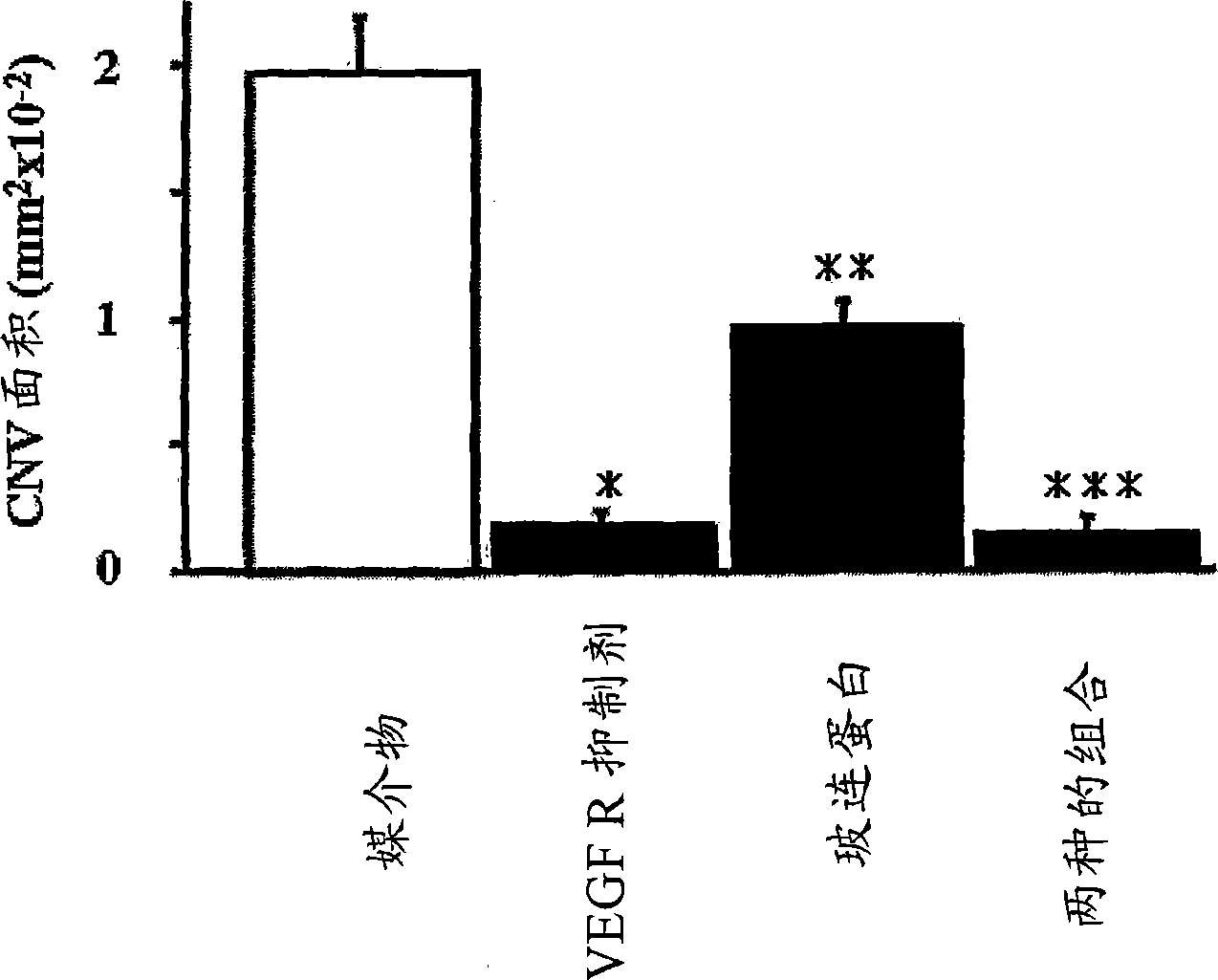
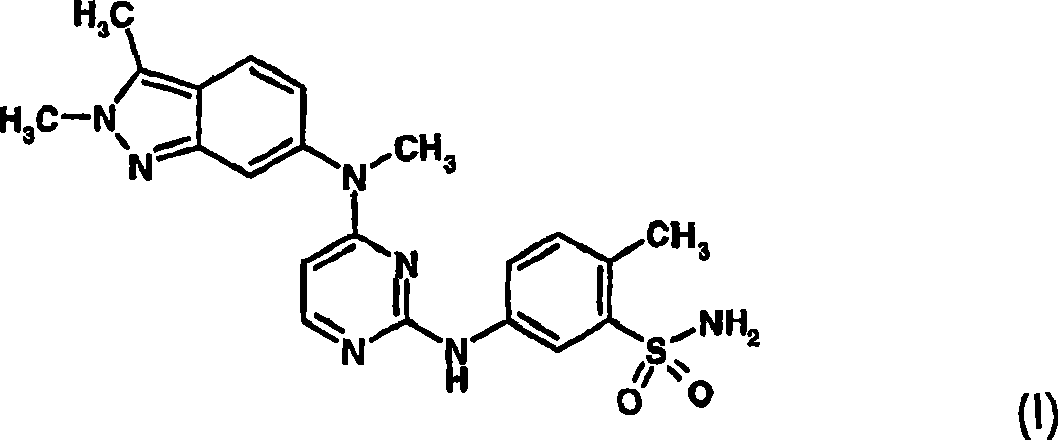
Treatment of ocular neovascular disorders such as macular degeneration, angiod streaks, uveitis and macular edema
A technology for neovascularization and macular edema, applied in cardiovascular system diseases, sensory diseases, organic active ingredients, etc., can solve problems such as progressive vision loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0089] As used herein, the symbols and conventions used in these Procedures, Schemes and Examples are consistent with those used in contemporary scientific literature, eg, the Journal of the American Chemical Society or the Journal of Biological Chemistry. Amino acid residues are generally designated using standard one-letter or three-letter abbreviations, and unless otherwise stated, these amino acids are assumed to be in the L-configuration. All starting materials were obtained from commercial suppliers and used without purification unless otherwise stated. Specifically, the following abbreviations are used in the examples and throughout the specification:
[0090] g (gram); mg (mg);
[0091] L (liter); mL (milliliter);
[0092]μl (microliter); psi (pounds per square inch);
[0093] M (mole); mM (millimole);
[0094] N (standard) Kg (kilogram)
[0095] i.v. (intravenous); Hz (hertz);
[0096] MHz (megahertz); mol (number of moles);
[0097] mmol (mmol); RT (room tempe...
Embodiment 2
[0133] Preparation of 2,3-dimethyl-6-amino-2H-indazole:
[0134]
[0135] method 1:
[0136] To a stirred solution of 2,3-dimethyl-6-nitro-2H-indazole (1.13 g) in 2-methoxyethyl ether (12 ml) was added dropwise over 5 minutes at 0°C A solution of 4.48 g of tin(II) chloride in 8.9 ml of concentrated hydrochloric acid. After the addition was complete, the ice bath was removed and the solution was stirred for an additional 30 minutes. About 40ml of diethyl ether was added to the reaction, resulting in a precipitate. The resulting precipitate was isolated by filtration and washed with diethyl ether to give a yellow solid (1.1 g, 95%) as the hydrochloride salt of 2,3-dimethyl-2H-indazol-6-amine.
[0137] 1 H NMR (300MHz, DMSO-d 6 )δ 7.77(d, J=8.9Hz, 1H), 7.18(s, 1H), 7.88(m, 1H), 4.04(s, 3H), 2.61(s, 3H).MS(ES+, m / z) 162 (M+H).
[0138] Method 2:
[0139] A 2L, 3-neck round bottom flask was equipped with a nitrogen inlet and outlet and mechanical stirring. A moderate ni...
Embodiment 3
[0142] Preparation of N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine:
[0143]
[0144] method 1:
[0145] To the stirred intermediate Example 2 product (2.97 g, .015 mol) and NaHCO at room temperature 3 (5.05 g, .06 mol) in THF (15 mL) and ethanol (60 mL) was added 2,4-dichloropyrimidine (6.70 g, .045 mol). After the reaction was stirred at 85°C for 4 hours, the suspension was cooled to room temperature, filtered and washed well with ethyl acetate. The filtrate was concentrated under reduced pressure and the resulting solid was triturated with ethyl acetate to give N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine (89%, 3.84g).
[0146] 1 H NMR (400MHz, DMSO-d 6 )δ 7.28(d, J=9.0Hz, 1H), 6.42(d, J=8.8Hz, 1H), 6.37(s, 1H), 5.18(br s, 1H), 3.84(s, 3H), 2.43( s, 3H). MS (ES+, m / z) 274 (M+H)
[0147] Method 2:
[0148] To a 1 L 3-necked flask equipped with a pneumatic mechanical stirrer, thermometer and nitrogen inlet / outlet was added the product of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com