Orally taken or buccal antiviral medicament-Ribavirin tablet and method of preparing the same
A production method, the technology of Virin tablets, is applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as high production costs, restrictions on the use of dosage forms, and high components of ribavirin, and achieve Rapid disintegration, rapid absorption, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
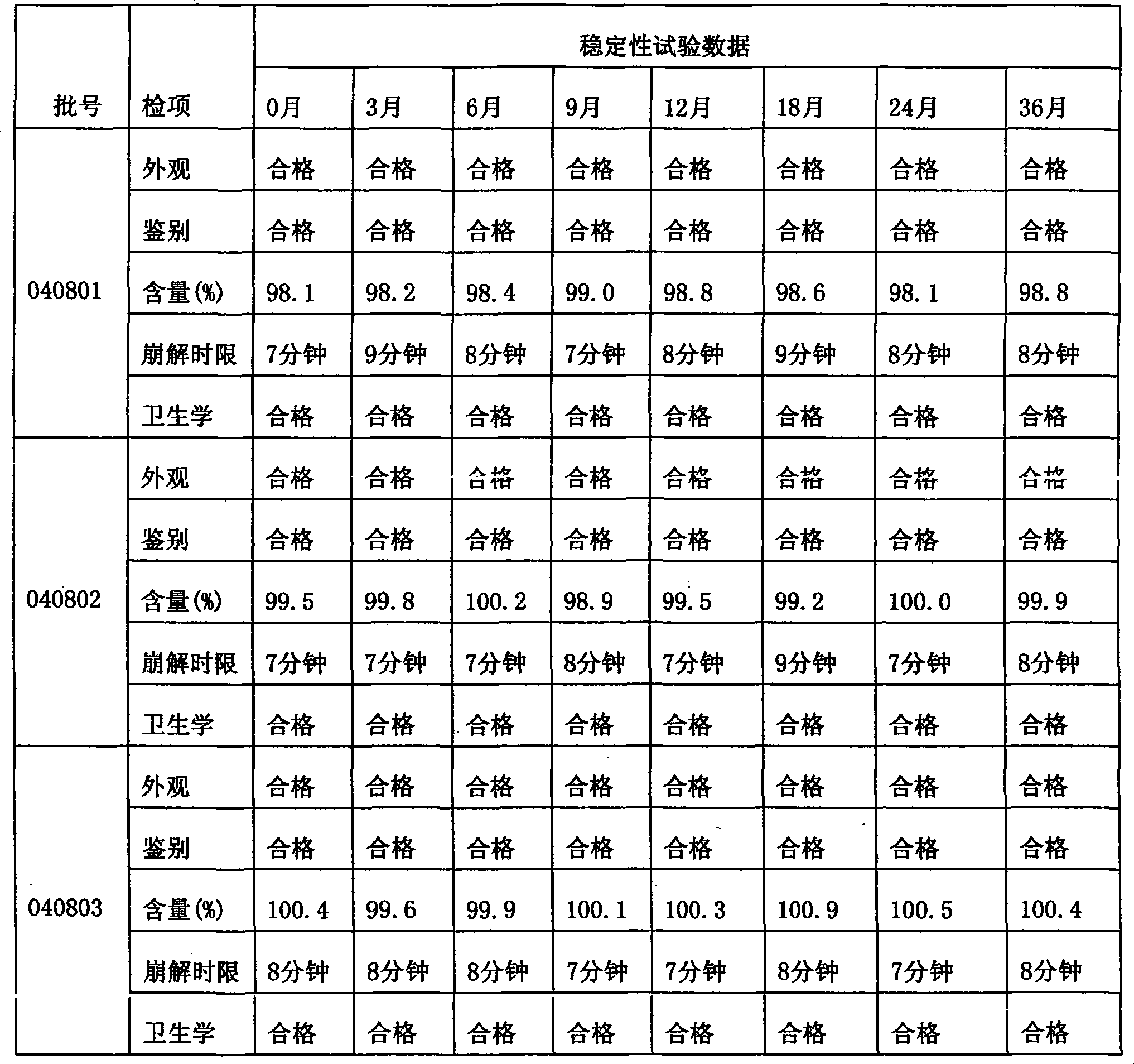
Image
Examples
Embodiment 1
[0027] 1. Ribavirin tablet (20mg / tablet) formula:
[0028] Name of main and auxiliary materials per 1000 tablets (g)
[0029] Ribavirin 20g (converted to pure amount)
[0030] Sucrose 435g
[0031] Dextrin 15g
[0032] Starch 20g
[0033] 5g citric acid (dissolved in binder)
[0034] Microcrystalline Cellulose 5g
[0035] Sodium Lauryl Sulfate 2.5g (dissolved in binder)
[0036] Hydroxypropyl methylcellulose 1g (add appropriate amount of purified water to make 2% binder)
[0037] Low-substituted hydroxypropyl cellulose 10g
[0039] 2. The preparation method is: take 80 mesh ribavirin, 80 mesh sucrose and other 100 mesh excipients (dextrin, starch, microcrystalline cellulose; low-substituted hydroxypropyl cellulose) and mix, first add and dissolve Stir the 2% hydroxypropyl methylcellulose solution of sodium lauryl sulfate, then add the 2% hydroxypropyl methylcellulose solution dissolved in citric acid to make soft material, and pass throu...
Embodiment 2
[0041] 1. Ribavirin tablet (50mg / tablet) formula:
[0042] Names of main and auxiliary materials per 1000 tablets (g)
[0043] Ribavirin 50g (converted to pure amount)
[0044] Sucrose 405g
[0045] Dextrin 15g
[0046] Starch 20g
[0047] 5g citric acid (dissolved in binder)
[0048] Microcrystalline Cellulose 5g
[0049] Sodium Lauryl Sulfate 2.5g (dissolved in binder)
[0050] Hydroxypropyl methylcellulose 1g (add appropriate amount of purified water to make 2% binder)
[0051] Low-substituted hydroxypropyl cellulose 10g
[0053] 2. The preparation method is: take 80 mesh ribavirin, 80 mesh sucrose and other 100 mesh excipients (dextrin, starch, microcrystalline cellulose, low-substituted hydroxypropyl cellulose) and mix, first add and dissolve Stir the 2% hydroxypropyl methylcellulose solution of sodium lauryl sulfate, then add the 2% hydroxypropyl methylcellulose solution dissolved in citric acid to make soft material, and pass throug...
Embodiment 3
[0055] 1. Ribavirin tablet (100mg / tablet) formula:
[0056] Name of main and auxiliary materials per 1000 tablets (g)
[0057] Ribavirin 100g (converted to pure amount)
[0058] Sucrose 374g
[0059] Dextrin 15g
[0060] Starch 5g
[0061] 5g citric acid (dissolved in binder)
[0062] Sodium carboxymethyl starch 5g
[0063] Sodium Lauryl Sulfate 2g (dissolved in binder)
[0064] Hydroxypropyl methylcellulose 1g (add appropriate amount of purified water to make 2% binder)
[0065] Low-substituted hydroxypropyl cellulose 10g
[0067] 2. The preparation method is: take 80-mesh ribavirin, 80-mesh sucrose and other 100-mesh excipients (dextrin, starch, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose) and mix, first add and dissolve Stir the 2% hydroxypropyl methylcellulose solution of sodium lauryl sulfate, then add the 2% hydroxypropyl methylcellulose solution dissolved in citric acid to make a soft material, pass throug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com