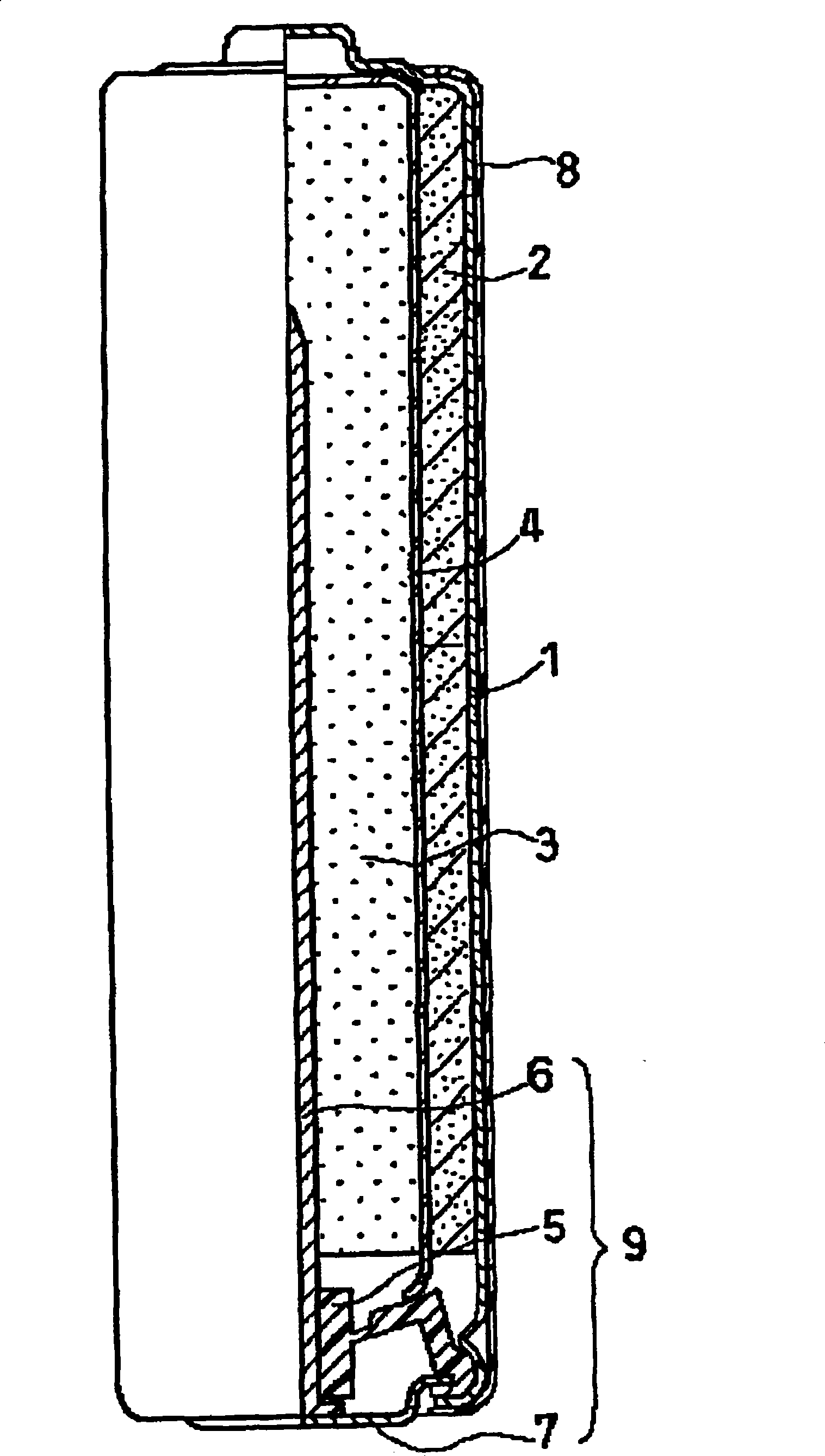
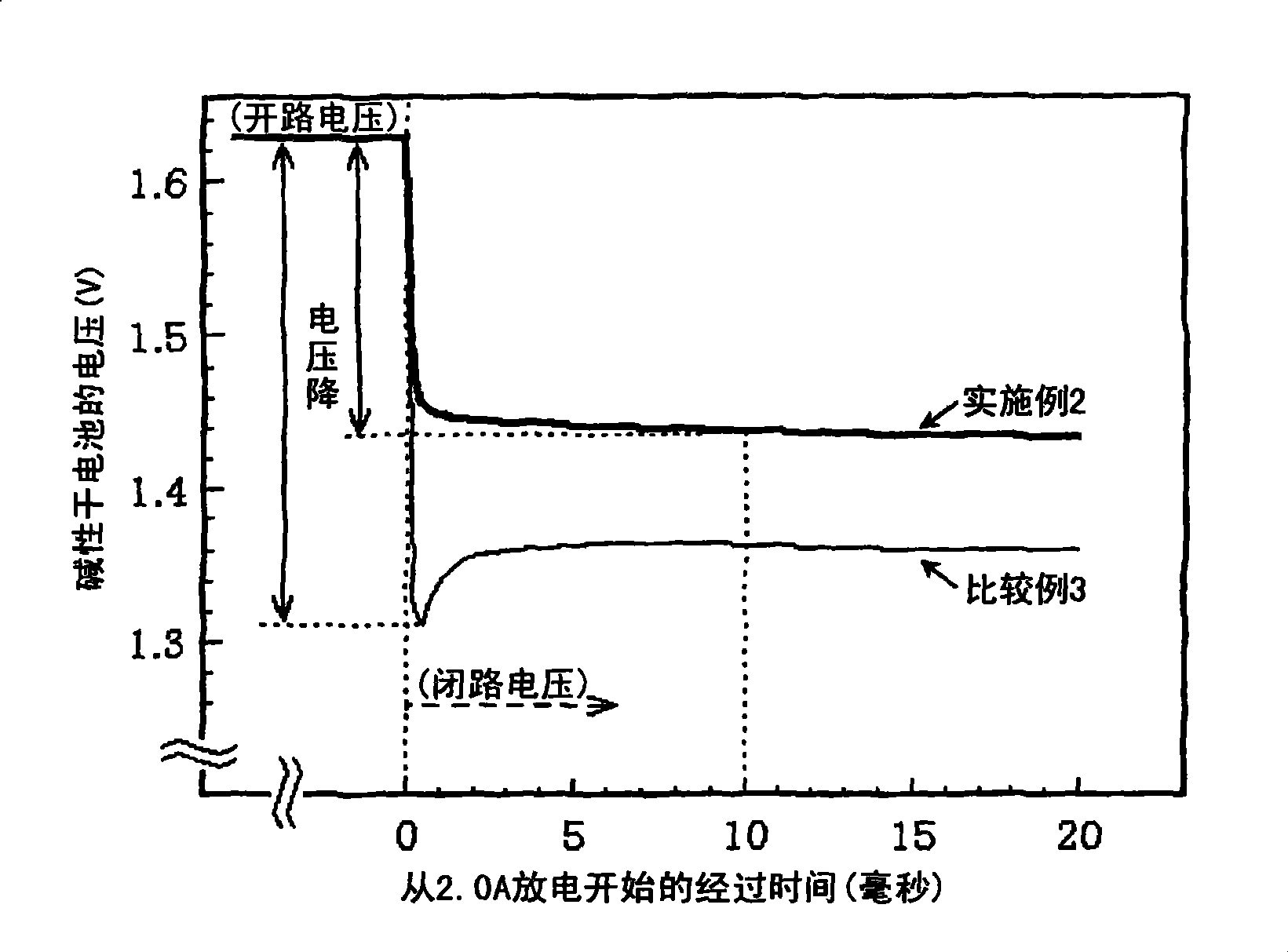
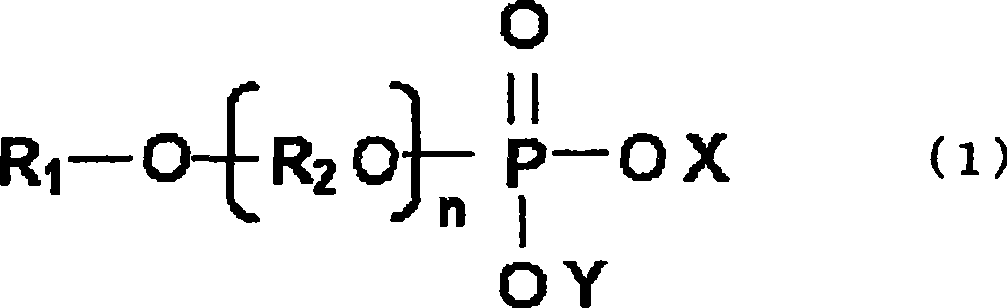Alkaline dry battery
A dry battery and alkaline technology, which is applied in the field of alkaline dry batteries, can solve the problems of reduced negative electrode active material filling, hindered electrode reaction, and reduced negative electrode density, achieving excellent leakage resistance and discharge performance, and the effect of suppressing gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9 and comparative example 1~3
[0129] (1) Fabrication of the battery case
[0130] Prepare a ring-shaped cold-rolled steel sheet with a thickness of 0.4 mm, go through the electroplating process with the conditions shown in Table 1, and use a protective gas composed of 6.5% hydrogen and 93.5% nitrogen at a dew point of -40 ° C, and at 550 ° C Perform an annealing process for 6 hours at the soaking temperature to form nickel-containing layers on both sides of the steel sheet, thereby obtaining a nickel-plated steel sheet.
[0131] Table 1
[0132]
[0133] The above-mentioned nickel-plated steel plate is punched into a circle, and then processed into a cup-shaped intermediate product. Then, the cup-shaped intermediate product is made into a can by the DI method of continuously performing drawing processing by two drawing dies and reducing extrusion processing by three reducing extrusion dies, and the outer diameter is 13.90mm. mm, and the thickness of the side is 0.18mm battery case 1. In the center of...
Embodiment 10~17 and comparative example 4~5
[0167] The compound represented by general formula (2), that is, the phosphoric acid ester represented by following general formula (5) is obtained by esterification reaction of alkyl alcohol and phosphoric acid.
[0168] Formula (5)
[0169]
[0170] At this time, the number of carbon atoms of the alkyl group in the alkyl alcohol and the kind of salt that neutralizes the phosphoric acid group are changed. That is, compounds 12 to 21 were produced by varying m, n, m+n, X, and Y, which are the structural parameters of the general formula (5), as shown in Table 3.
[0171] Compounds 12 to 21 were added to the negative electrode, and AA-shaped alkaline dry batteries were produced in the same manner as in Example 1, and evaluated in the same manner as above. The results of these evaluations are shown in Table 3.
[0172] table 3
[0173]
[0174] In the batteries of Comparative Examples 4 and 5, the number of carbon atoms in the phosphate molecule was increased, which hin...
Embodiment 18~23
[0177] By the alkylation reaction of phosphoric acid ester, the compound represented by General formula (3), ie, the phosphoric acid ester represented by following General formula (6) is obtained.
[0178] Formula (6)
[0179]
[0180] At this time, by changing the number of carbon atoms in the alkyl group and the type of salt that neutralizes the phosphoric acid group, the structural parameters of the general formula (6), namely n, X, and Y, are variously changed as shown in Table 4, and the Compounds 22-27 were obtained.
[0181] Compounds 22 to 27 were added to the negative electrode, and the same method as in Example 1 was used to fabricate single-size alkaline dry batteries, respectively, and evaluated in the same manner as above. These evaluation results are shown in Table 4.
[0182] Table 4
[0183]
[0184]The batteries of Examples 18-23 of the present invention where n=1-6 in the general formula (6) have less voltage drop, exhibit good discharge performance ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com