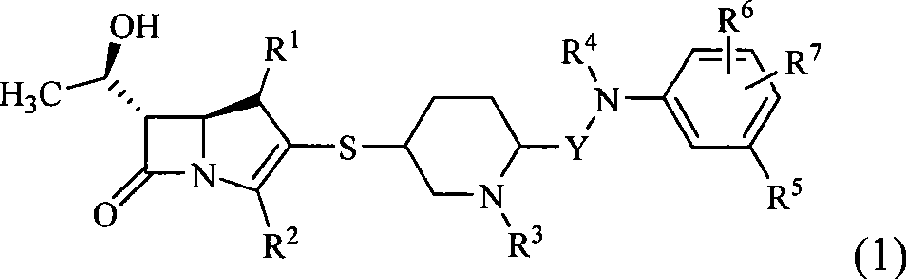
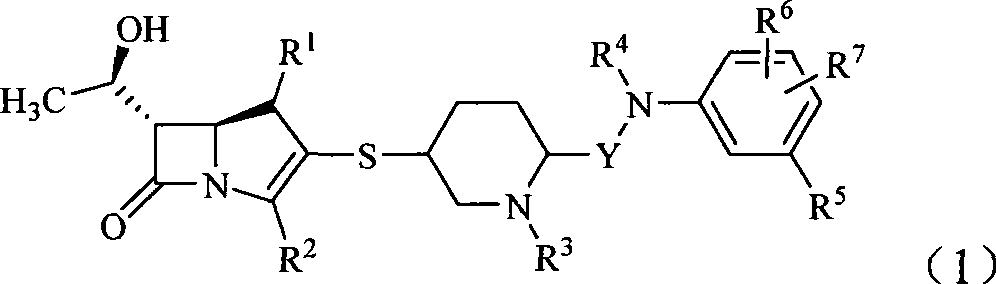
Carbapenem antibiotic containing sulfhydryl piperidine
A representative, methyl-based technology, applied in the field of medicine, can solve the problems of low clinical availability, increased bacterial resistance, and inability to meet clinical needs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 Preparation of 1-tert-butoxycarbonyl-5-methanesulfonyloxypiperidine-2-methanol
[0111] In the dry reaction flask, add 100ml of dioxane, then add 6.8g (50mmol) of anhydrous zinc chloride, 5.4g (100mmol) of potassium borohydride, stir at room temperature for 1h, then add 1-tert-butoxycarbonyl-5- 8.4 g (25 mmol) of methyl methanesulfonyloxypiperidine-2-carboxylate. The temperature was slowly raised to reflux under stirring for 2h. Cool to room temperature, add 100ml of 1mol / L hydrochloric acid to dilute, and extract with ethyl acetate. The organic phase was washed with saturated brine, dried and concentrated to obtain 6.6 g of 1-tert-butoxycarbonyl-5-methanesulfonyloxypiperidine-2-methanol, yield: 85.3%.
Embodiment 2
[0112] Example 2 Preparation of 5-acetylthio-1-tert-butoxycarbonylpiperidine-2-methanol
[0113] In the dry reaction flask, add 15.5g (50mmol) of 1-tert-butoxycarbonyl-5-methanesulfonyloxypiperidine-2-methanol and 9.2g (100mmol) of potassium thioacetate, then add DMF150ml, stir and heat until dissolved Afterwards, heat preservation reaction 10h. After cooling to room temperature, dilute with 200ml of water, extract with ethyl acetate, wash the organic phase with water, dry and concentrate to obtain 13.4g of the product, yield: 92.5%.
Embodiment 3
[0114] Example 3 Preparation of 5-mercapto-2-[N-tert-butoxycarbonyl-N-(3-benzoic acid)amino]methylene-1-(tert-butoxycarbonyl)piperidine prepare
[0115] Under the protection of nitrogen, add 5.8g (20mmol) of 5-acetylthio-1-tert-butoxycarbonylpiperidine-2-methanol, 100ml of dichloromethane, drop into 4ml of TMSI (30mmol), stir vigorously at room temperature for 4h, ice Cool in a water bath to 0°C, stir for 30 minutes, and filter under reduced pressure. The filter cake was washed with a small amount of dichloromethane, and the filtrate was collected in a precooled flask. Under the protection of nitrogen at 0°C, a solution of 4.7g (21mmol) 3-tert-butoxycarbonylamino-benzoic acid in 20ml triethylamine / tetrahydrofuran (1:4, v / v) was added dropwise to the solution. , reacted at 0-5°C for 4h, decolorized the reaction solution, filtered it with suction, concentrated the filtrate to dryness under reduced pressure, then added 50ml of 5mol / L hydrochloric acid, stirred and reacted at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com