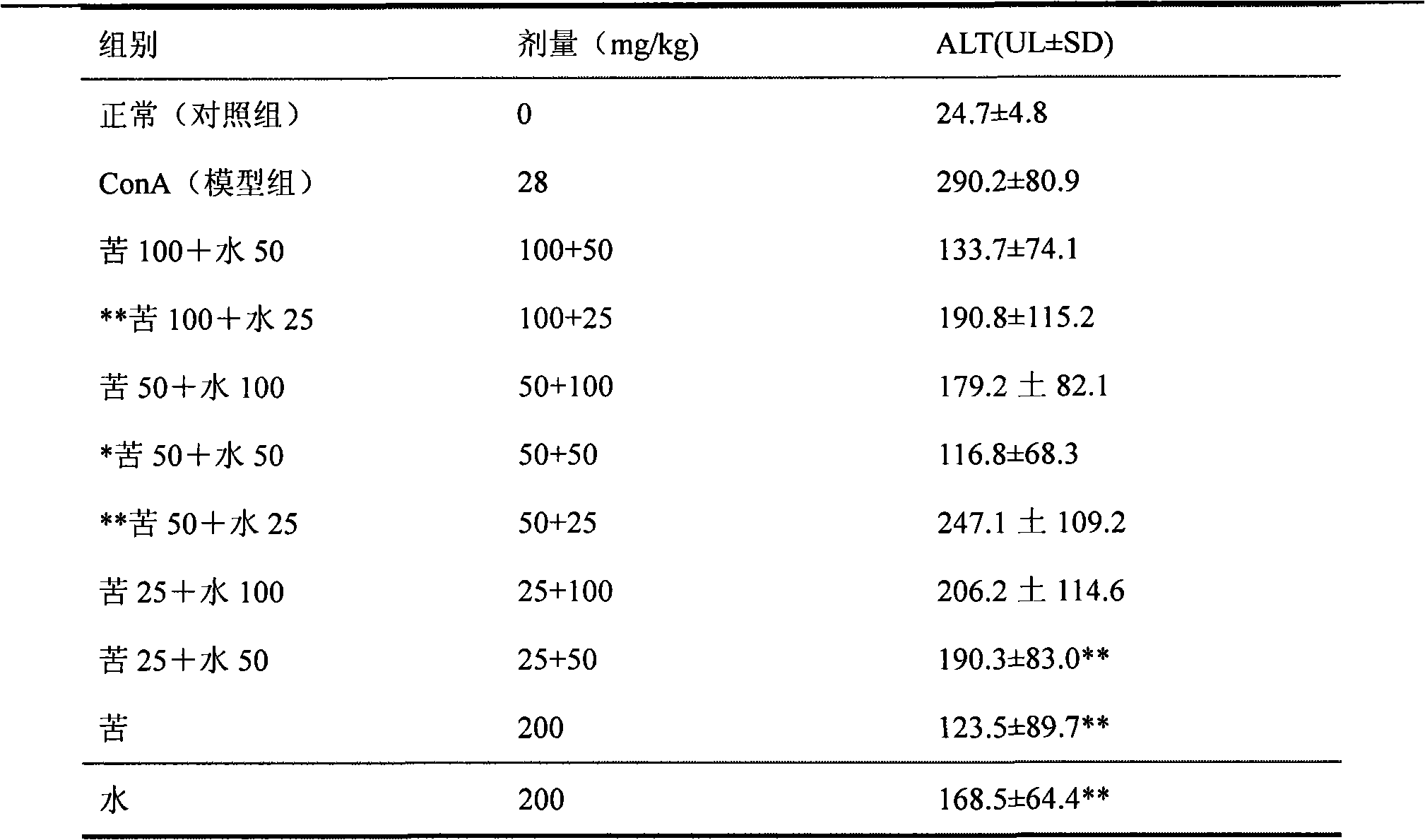
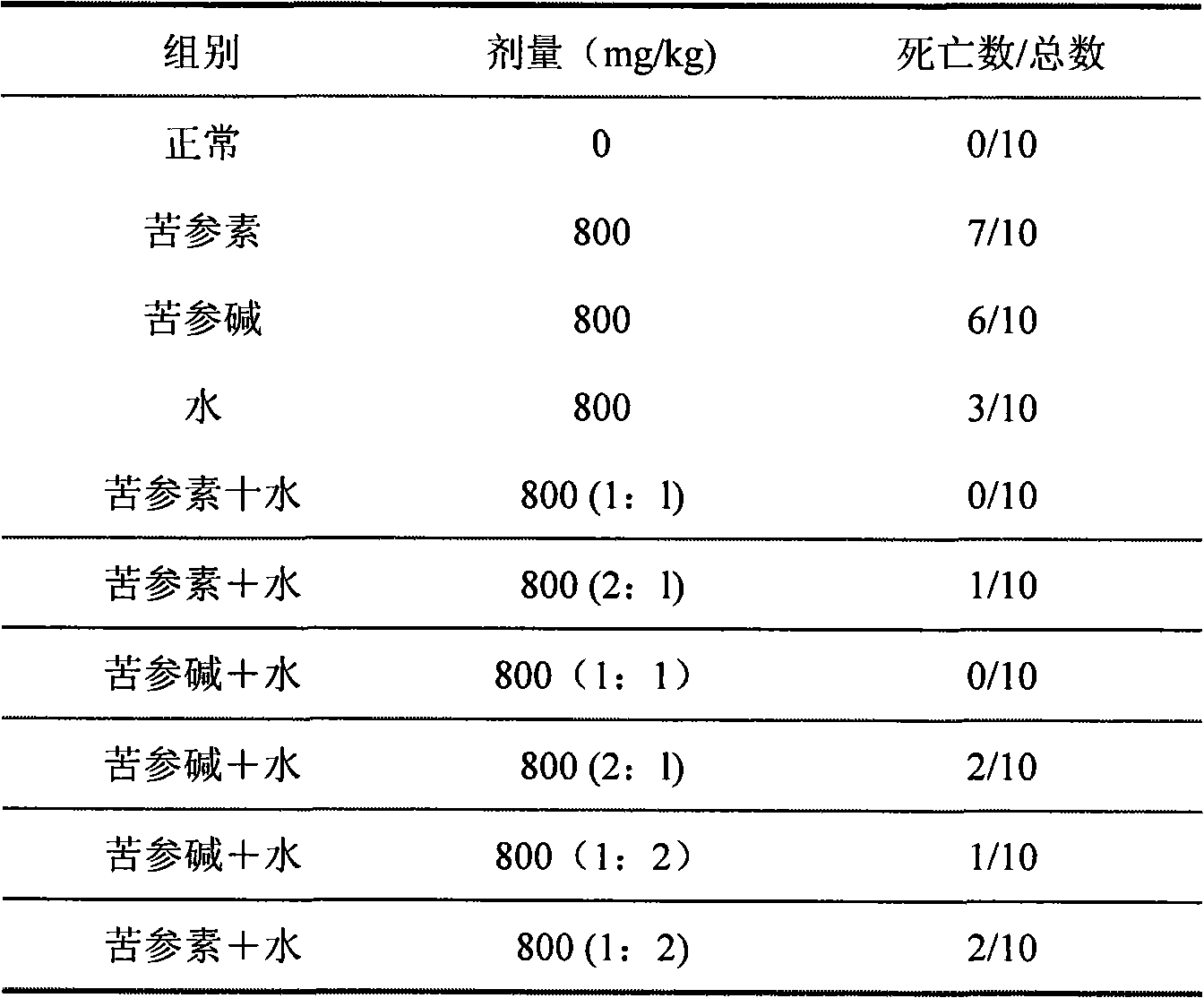
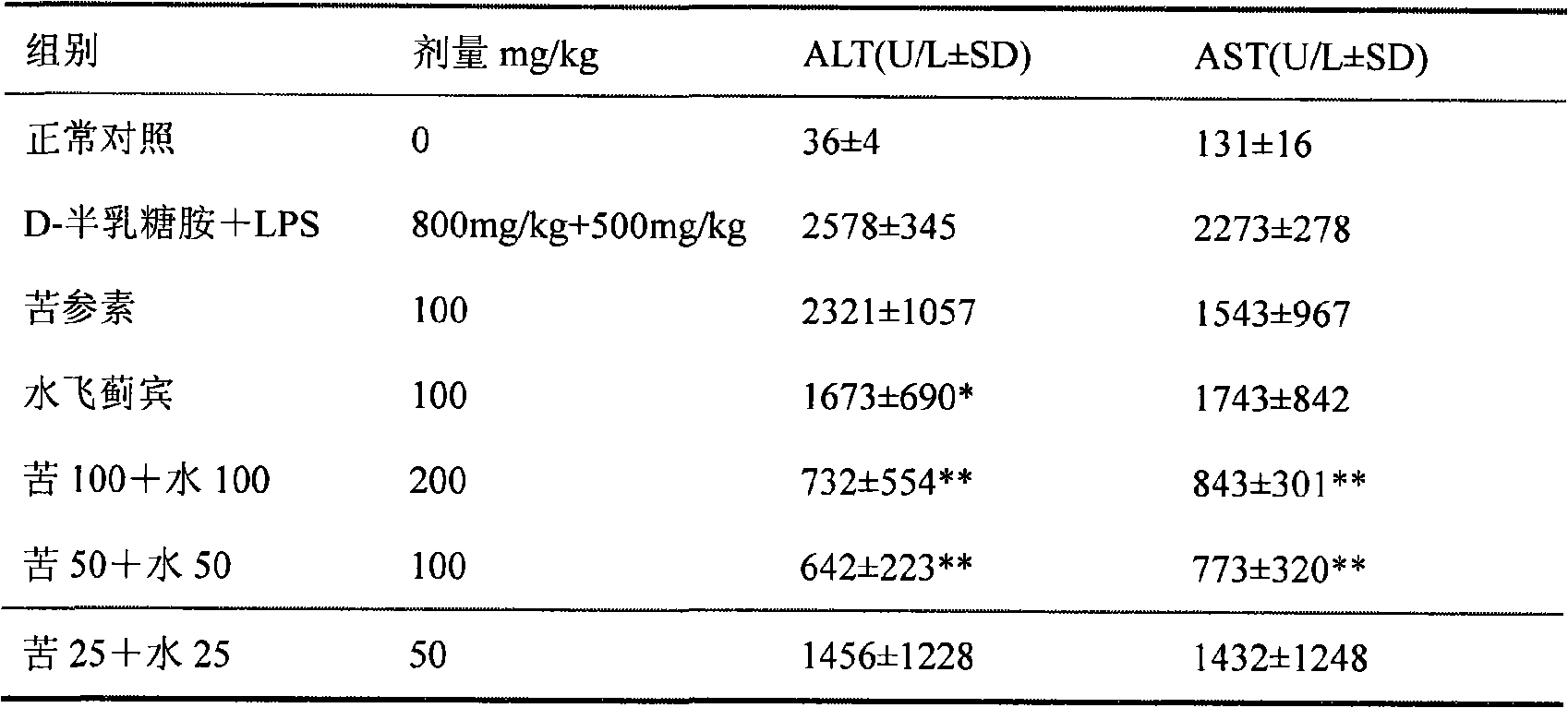
Medicine composition containing silymarin and kurainone or matrine and use thereof
A technology of silybin and matrine, which is applied in the treatment of liver diseases and diseases, and can solve problems such as large side effects, adverse family effects, and long treatment courses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Matrine 100mg
[0048] Silibinin 100mg
[0049] Microcrystalline Cellulose 13.0mg
[0050] Appropriate amount of lactose
[0051] Magnesium Stearate Appropriate amount
[0052]
[0053] 237.5mg per tablet
[0054] After uniformly mixing the above-mentioned raw materials and auxiliary materials, it is wet granulated according to the conventional method, dried and pressed into tablets.
Embodiment 2
[0056] Matrine 50mg
[0057] Silibinin 50mg
[0058] NaCl 0.9g
[0059] Appropriate amount of water for injection
[0060]
[0061] 100ml each
[0062] Take sodium chloride, stir and dissolve with water for injection, then add matrine and silibinin respectively, continue to stir to dissolve completely, add water for injection to the total amount, filter until clear, potting, and sterilize to obtain.
Embodiment 3
[0064] Pill Core Prescription
[0065] Matrine 150g
[0066] Silybin 150g
[0067] Microcrystalline Cellulose 15g
[0068] Hypromellose 5g
[0069] Pure water 200ml
[0070]
[0071] Makes 1000 capsules
[0072] Coating prescription
[0073] 25% based cellulose aqueous dispersion 184g
[0074] Pure water 123g
[0075]
[0076] Makes 1000 capsules
[0077] Microcrystalline cellulose, matrine, and silybin were crushed through 80-mesh sieve in advance, weighed according to the prescription of pill 1, mixed evenly, and hydroxypropyl methylcellulose aqueous solution was used as a binder to make micropills. Dry at 50-60°C, select pellets of 20-30 mesh, and set aside. Put the prepared and selected pellets in a fluidized bed, adopt the bottom spray method, and suspend and fluidize them by hot air. Provide the coating solution at a rate of 5 g slurry per minute, atomize at 2 bar, and start spraying the fluidized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com