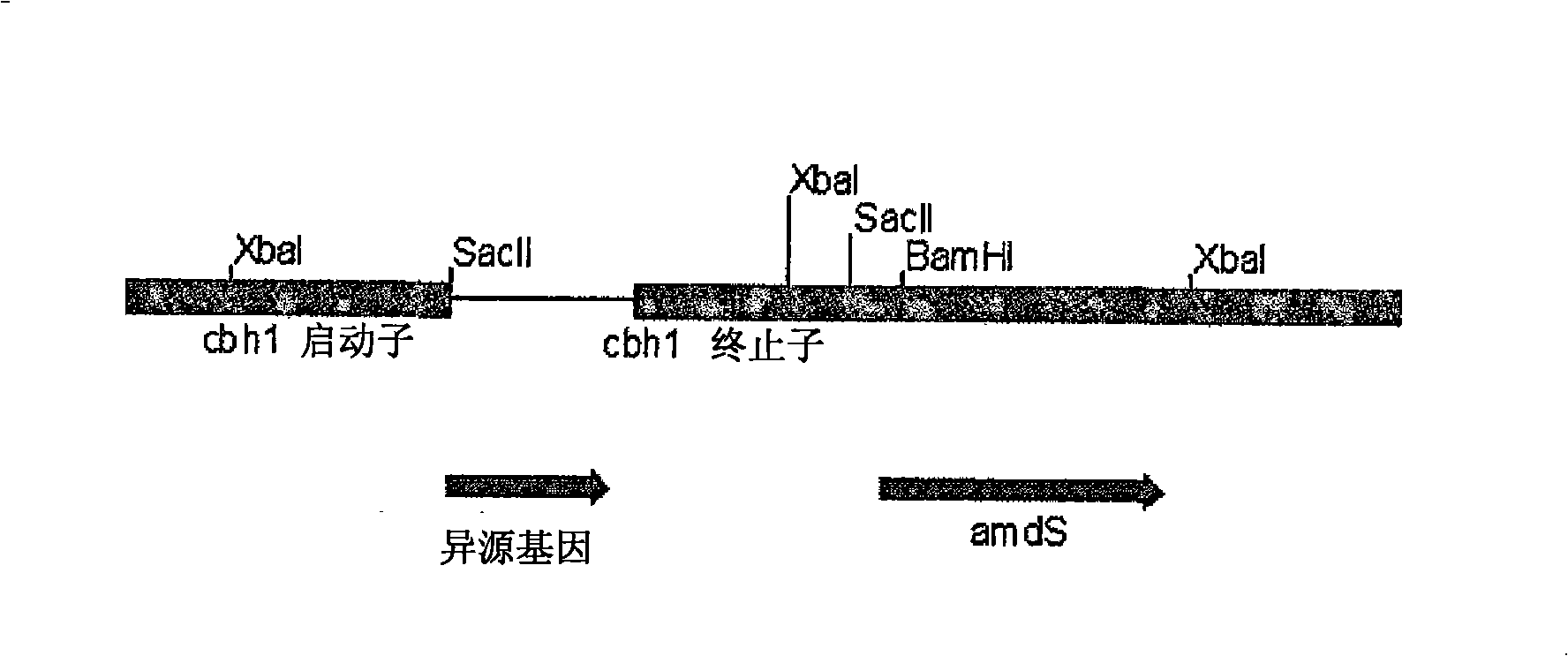
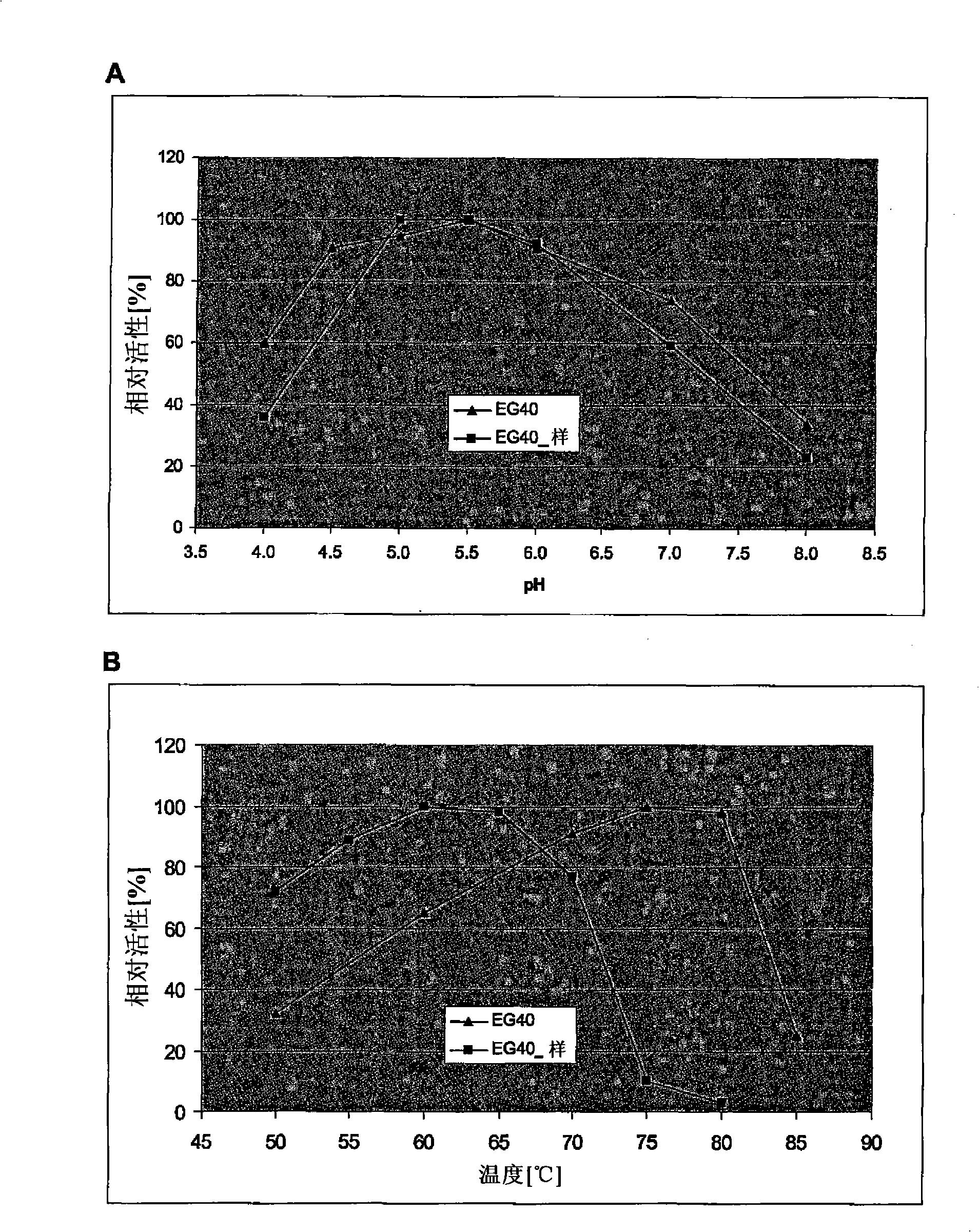
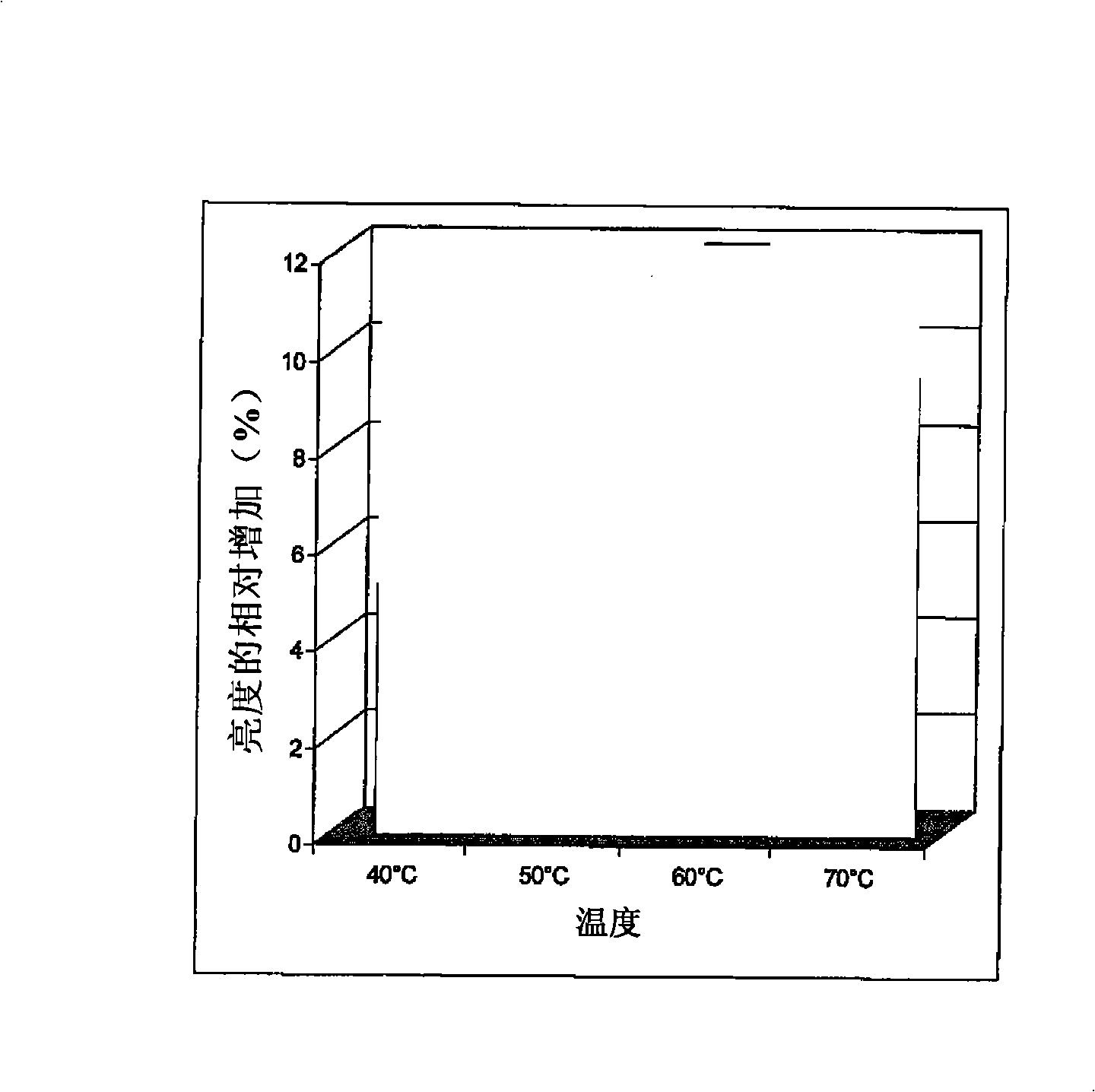
Novel enzymes
An endoglucanase and sequence technology, applied in the directions of enzymes, hydrolases, glycosylases, etc., can solve the problems of reducing the strength of fabrics and increasing the burden of washing equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Embodiment 1, the cultivation of acremonium thermophile ALKO4245
[0122] Acremonium thermophila strain ALKO4245 in a 2 liter bioreactor (Braun Biostat B, Braun, Melsungen, Germany) were grown in g / l in the following media: Solka Floc cellulose 40, corn extract powder 15, brewers' used grain 5, oat xylan 3, locust bean gum 3 , (NH 4 ) 2 SO 4 5 and KH 2 PO 4 5. The pH range is 5.2±0.2(NH 3 / H 2 SO 4 ), ventilation 1vvm, stirring 300-600rpm, with Struktol Defoaming control, temperature 42°C. The culture time is 4 days. After incubation, cells and other solids are collected by centrifugation, and the supernatant is recovered.
Embodiment 2
[0123] Example 2. Purification of endoglucanase from Acremonium thermophila ALKO4245
[0124] The culture supernatant of Acremonium thermophila ALKO4245 grown as described in Example 1 was incubated at 70° C. for 24 hours after concentration by ultrafiltration. Pure endoglucanase was obtained by sequential purification using hydrophobic interaction and cation exchange chromatography followed by gel filtration. The endoglucanase activity of the fractions collected during the purification was determined using carboxymethylcellulose (CMC) as substrate (according to the method of IUPAC, 1987).
[0125] The concentrated culture supernatant was added to a HiPrep 16 / 10 Butyl FF hydrophobic interaction column (GE Healthcare) containing 1M (NH 4 ) 2 SO 4 Equilibrate with 20 mM potassium phosphate buffer at pH 6.0. Bound protein was eluted with a linear gradient from the above buffer to 5 mM potassium phosphate buffer, pH 6.0. Fractions were collected and assayed for endoglucanase ...
Embodiment 3
[0132] Cloning of the cel45A and cel45B genes of embodiment 3, Acremonium thermophila (ALKO4245)
[0133] Standard molecular biology methods are used in the isolation and enzymatic treatment of DNA (plasmids, DNA fragments), transformation of E. coli, and the like. The basic methods used are described in standard molecular biology handbooks, eg Sambrook, J. et al., 1989 and Sambrook J. and Russell, D.W., 2001.
[0134] In Lambda DASH A genomic library of Acremonium thermophila ALKO4245 was constructed in II vector (Stratagene, USA) according to the manufacturer's instructions. Chromosomal DNA was isolated by the method of Raeder and Broda, 1985 and partially digested with Sau3A. Digested DNA was size fractionated on an agarose gel and fragments of selected size (approximately 5-23 kb) were isolated, dephosphorylated, and ligated into BamHI digested vector arms. The ligation mix was packaged using Gigapack III Gold pack extract according to the manufacturer's instructions (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com