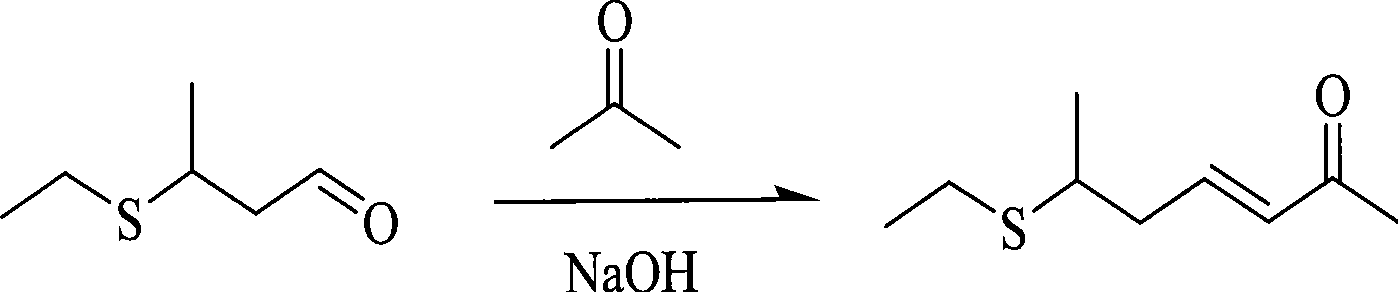
Process for synthesizing 6-ethyl mercapto-3-heptylene-2-ketone
A synthesis method and ethylthio-based technology are applied in the preparation of thioethers, organic chemistry, etc., and can solve the problems of complicated operation, expensive post-processing, and many steps, and achieve the effects of good atom economy, low cost, and simplified steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Sodium hydroxide catalyzed the synthesis of 6-ethylthio-3-hepten-2-one.
[0012] Mix 15ml of acetone (0.2mol, 11.6g) and 45ml of 1.5% aqueous sodium hydroxide solution (wherein the quality of sodium hydroxide is 0.68g, 0.017mol) and place in a thermometer, reflux condenser, constant pressure dropping funnel In a 100ml three-necked flask, stir to make it evenly mixed, and heat to make it boil. In boiling state, slowly drop into the mixture of 3.90g of 3-ethylthiobutyraldehyde (0.03mol) and 10ml of acetone (0.13mol, 7.54g). Control the rate of addition, and the dropwise addition is completed in 2 hours. During the dropwise addition, the temperature is controlled so that the system is always in a boiling state. After the dropwise addition, the system was kept in a boiling state and reacted for 3 hours. The components of the TLC detection system no longer changed. The layers were separated, and the aqueous phase (lower layer) was washed with 3×10 ml of dichloromethane, a...
Embodiment 2
[0014] Sodium carbonate catalyzed the synthesis of 6-ethylthio-3-hepten-2-one.
[0015] 15ml of acetone (0.2mol, 11.6g) and 20ml of 5% sodium carbonate aqueous solution (wherein the quality of sodium carbonate is 1.00g, 0.009mol) are mixed and placed in a 100ml three-necked thermometer equipped with a reflux condenser and a constant pressure dropping funnel. In the flask, stir to mix well and heat to boil. In boiling state, slowly drop into the mixture of 3.90g of 3-ethylthiobutyraldehyde (0.03mol) and 10ml of acetone (0.13mol, 7.54g). Control the rate of addition, and the dropwise addition is completed in 2 hours. During the dropwise addition, the temperature is controlled so that the system is always in a boiling state. After the dropwise addition, the system was kept in a boiling state and reacted for 3 hours. The components of the TLC detection system no longer changed. The system was extracted with 3×10 ml of dichloromethane, and the organic phases (lower layer) were c...
Embodiment 3
[0017] Synthesis of 6-ethylthio-3-hepten-2-one catalyzed by low temperature potassium hydroxide.
[0018] Mix 15ml of acetone (0.2mol, 11.6g) and 4.5ml of 15% aqueous sodium hydroxide solution (wherein the quality of sodium hydroxide is 0.78g, 0.02mol) and place in a container equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel. In a 100ml three-necked flask, stir to make it evenly mixed, and cool to 15°C. At this temperature, slowly drop in a mixture of 3.90g 3-ethylthiobutyraldehyde (0.03mol) and 10ml acetone (0.13mol, 7.54g), and control The rate of addition was completed in 2 hours. Extract with 3×10ml dichloromethane, and combine the organic phases. Anhydrous sodium sulfate was dried overnight, and the solvent was evaporated under reduced pressure to obtain a mixture of crude product 6-ethylthio-3-hepten-2-one and 6-ethylthio-4-hydroxyl-2-heptanone, of which 6 -Ethylthio-4-hydroxy-2-heptanone as the main product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com