Process for preparing kryocide
A cryolite and product technology, applied in the field of cryolite production, can solve problems such as high production cost, environmental pollution, and different degrees, achieve good social value and economic value, alleviate environmental pollution problems, and reduce dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
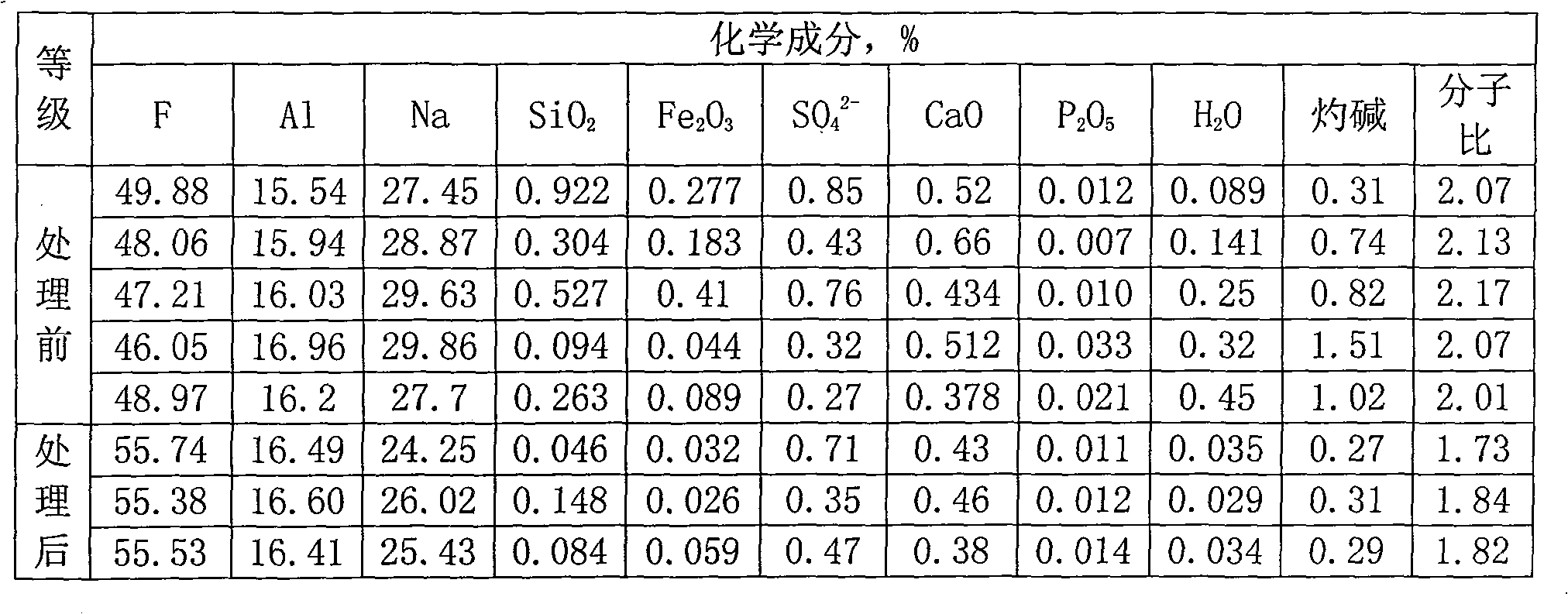
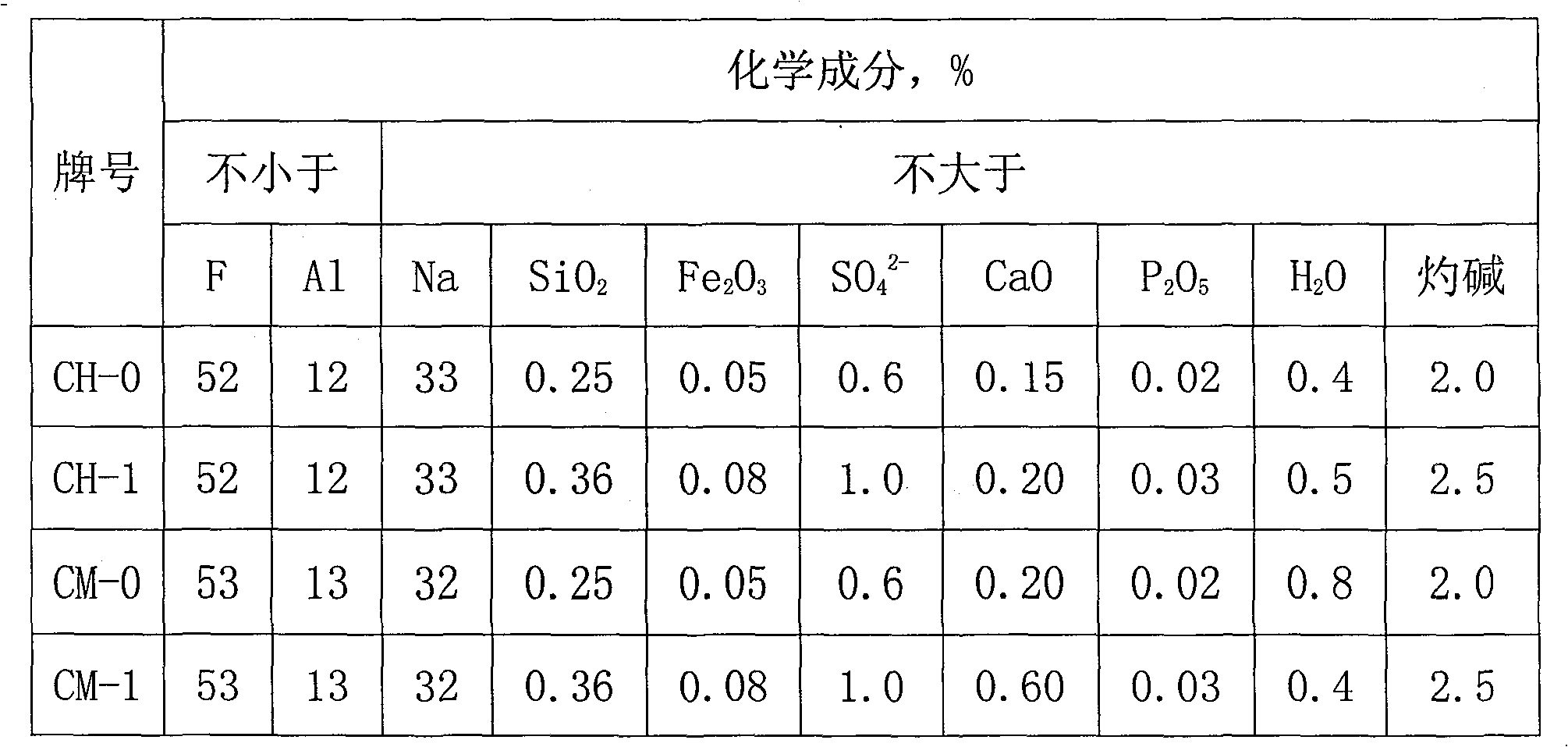
[0016] The production method of the present invention is as follows: use a jaw crusher or a hammer crusher to break the solid electrolyte block into small pieces of 30mm, and store them in the silo for later use; add the broken electrolyte block to a high-temperature melting furnace at 950°C , to generate molten electrolyte solution; the electrolyte solution is extracted with a vacuum extraction device to separate the solid waste from the pure electrolyte solution; after the electrolyte solution is cooled, it becomes an electrolyte block; the electrolyte block and sodium bifluoride are finely ground by a Raymond mill Finally, mix evenly according to the mass ratio of 4:1, put it into a high-temperature calcination furnace, and carry out a calcination reaction at a temperature of 550° C. to generate cryolite.
Embodiment 2
[0018] The production method of the present invention is as follows: use a jaw crusher or a hammer crusher to break the solid electrolyte block into small pieces of 40 mm, and store them in the silo for later use; decompose the broken pieces, and add them to a high-temperature melting furnace at 1000 ° C. In the process, a molten electrolyte solution is generated; the electrolyte solution is extracted with a vacuum extraction device to separate the solid waste from the pure electrolyte solution; the electrolyte solution is cooled to form an electrolyte block; the electrolyte block and sodium bifluoride are finely ground by a Raymond mill Finally, mix evenly according to the mass ratio of 6:1, put into a high-temperature calcination furnace to carry out calcination reaction at a temperature of 350° C., and generate cryolite.
Embodiment 3
[0020] The production method of the present invention is as follows: use a jaw crusher or a hammer crusher to break the solid electrolyte block into small pieces of 50 mm, and store them in the silo for later use; add the broken electrolyte block into a high-temperature melting furnace at 1100 ° C, Generate molten electrolyte; use a vacuum extraction device to extract the electrolyte to separate the solid waste from the pure electrolyte; the electrolyte is cooled to form an electrolyte block; the electrolyte block and sodium hydrogen fluoride are finely ground by a Raymond mill, Mix evenly according to the mass ratio of 8:1, put into a high-temperature calciner to carry out calcining reaction at a temperature of 150° C., and generate cryolite.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com