Improved process for preparing thieno-benzodiazepine compounds
A technology of compound and cyclization reaction, applied in the direction of organic chemistry, can solve the problems of high purity and preparation cost, high price of stannous chloride, influence, and unsatisfactory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
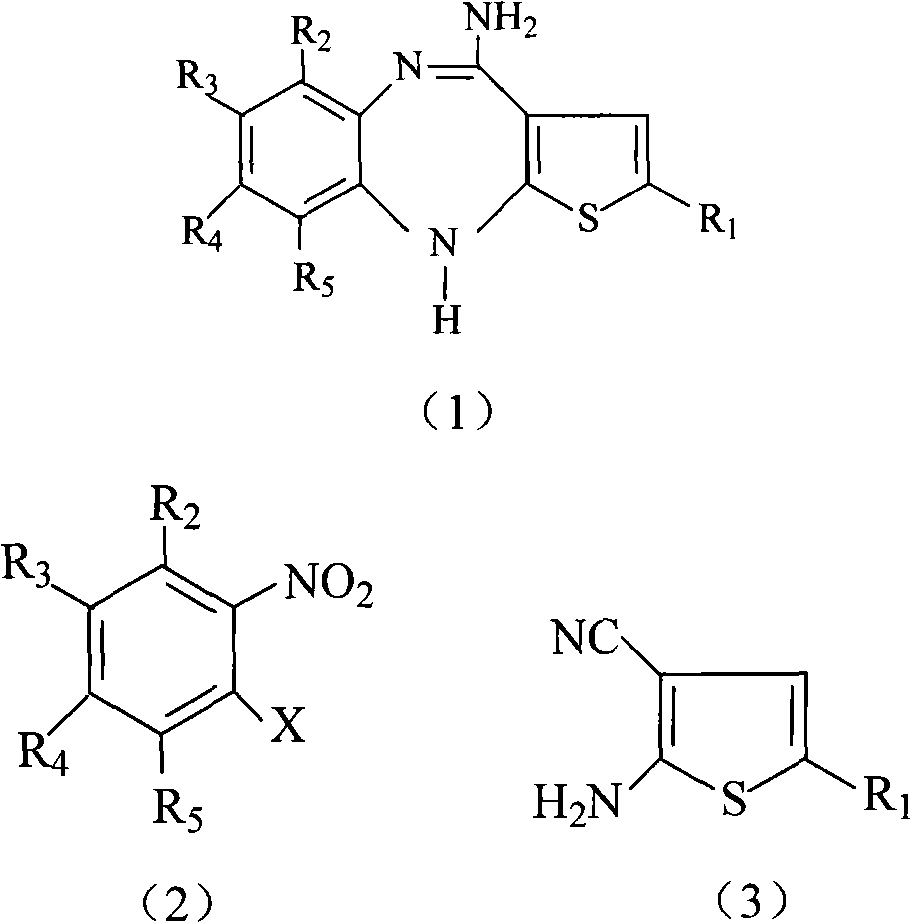
[0018] The reaction equation of the method for compound shown in the said preparation formula (1) or its salt of the present invention is as follows:
[0019]
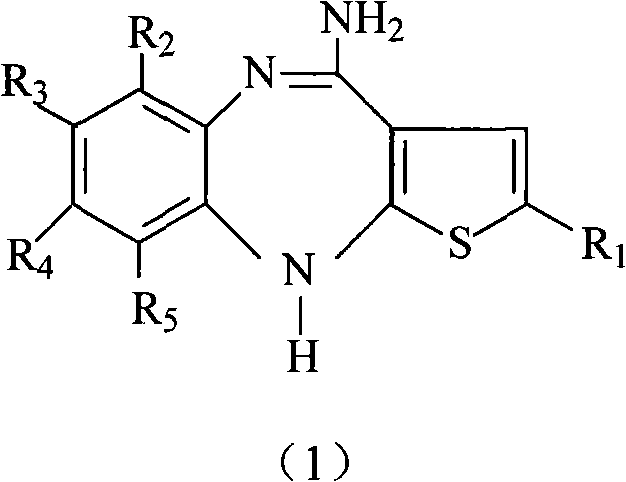
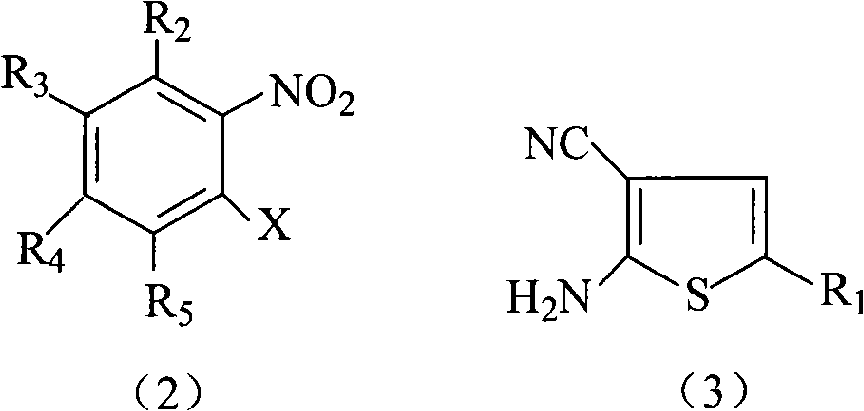
[0020] R in the reaction equation 1 , R 2 , R 3 , R 4 , R 5 and X have the same meanings as described above.
[0021] Said preparation method comprises the steps:
[0022] (a) the compound shown in formula (2) and the compound shown in formula (3) (see WO 2004094390 or US.Pat.No.5,229,382 for its preparation) in a phase transfer catalyst (quaternary ammonium salt compound, such as triethylbenzyl Ammonium chloride, etc.) and alkali (potassium or sodium carbonate, potassium or sodium hydroxide, or potassium or sodium hydride), with an aprotic polar solvent as the reaction solvent, at 0°C React at ~50°C for 1 hour to 10 hours to obtain the compound shown in formula (4), and recover the unreacted compound shown in formula (3) (the recovered compound shown in formula (3) can be recycled);
[0023] (b) react the co...
Embodiment 1
[0028] Potassium hydroxide (52.8g, 0.94mol), triethylbenzyl ammonium chloride (0.85g) and DMF (50ml) were successively added to a three-necked flask, and o-fluoronitrobenzene was added dropwise within 30 minutes at 25°C. (63.8g, 0.45mol), 2-amino-5-methylthiophene-3-carbonitrile (52g, 0.38mol) (see US. Hour. Pour the reaction solution into 400ml of crushed ice, stir the dark brown solid to precipitate out, and use CH 2 Cl 2 (200ml×3) extraction, and then CH 2 Cl 2 (200ml×2) to wash the aqueous layer. The organic phases were combined, washed with 100 ml of water, adjusted to pH 8-9 with 2N HCl solution, and finally washed with 400 ml of water. After separating the aqueous phase, the CH 2 Cl 2 Evaporate, recover the unreacted o-fluoronitrobenzene and use it mechanically, finally recrystallize with 200ml of absolute ethanol, and dry to obtain 81.3g of reddish-brown compound [2-(2-nitrophenylamino)-5-methylthiophene -3-carbonitrile], yield 83.3%, m.p.108.3℃~109℃.
[0029]...
Embodiment 2~15
[0031] Different products can be obtained with different raw materials and different catalysts with reference to Example 1, and the specific structure is shown in Table 1:
[0032] Table 1
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com