Self-assembly short peptide and use thereof in antineoplastic medicine preparation
A technology for self-assembly of short peptides and drugs, applied in antitumor drugs, drug combinations, peptides, etc., can solve the problems of no in vivo animal experiments, weak killing effect, and weakened curative effect, and achieve obvious social and economic benefits. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of self-assembled short peptide R418
[0029] 1. Reagents
[0030]PyBOP (benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate), Boc-Phe-Merrifield resin, hexahydropyridine, lutidine, TFA (trifluoroacetic acid), HPLC methanol, Protected amino acids (Fmoc-Lys(Boc)-OH, Fmoc-Ala-OH, Fmoc-Leu-OH, Fmoc-His(Trt)-OH, Fmoc-Phe-OH, Fmoc-Pro-OH, Fmoc-Ile-OH , Fmoc-Trp-OH, Fmoc-Gly-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Arg(Pmc)-OH.IPE), thioanisole (thioanisole), EDT (ethanedithiol), TIS (methyl ethyl sulfide), HOBT (1-hydroxybenzotriazole) are products of Merck; DMF (dimethylformamide) is a product of Samsung, South Korea; NMM (methylmafeline) is purchased from Sigma; DCM (two Chloromethane), phenol, and triethylamine are products of China Pharmaceutical (Group) Shanghai Chemical Reagent Company; methanol is a product of Shanghai Zhenxing Chemical Factory No. 1; tetrahydrofuran is a product of Shanghai Chemical Reagent Station Central Chemical Fact...
Embodiment 2
[0052] Example 2: High Performance Liquid Chromatography and Mass Spectrometry Detection of Self-Assembled Short Peptide R418
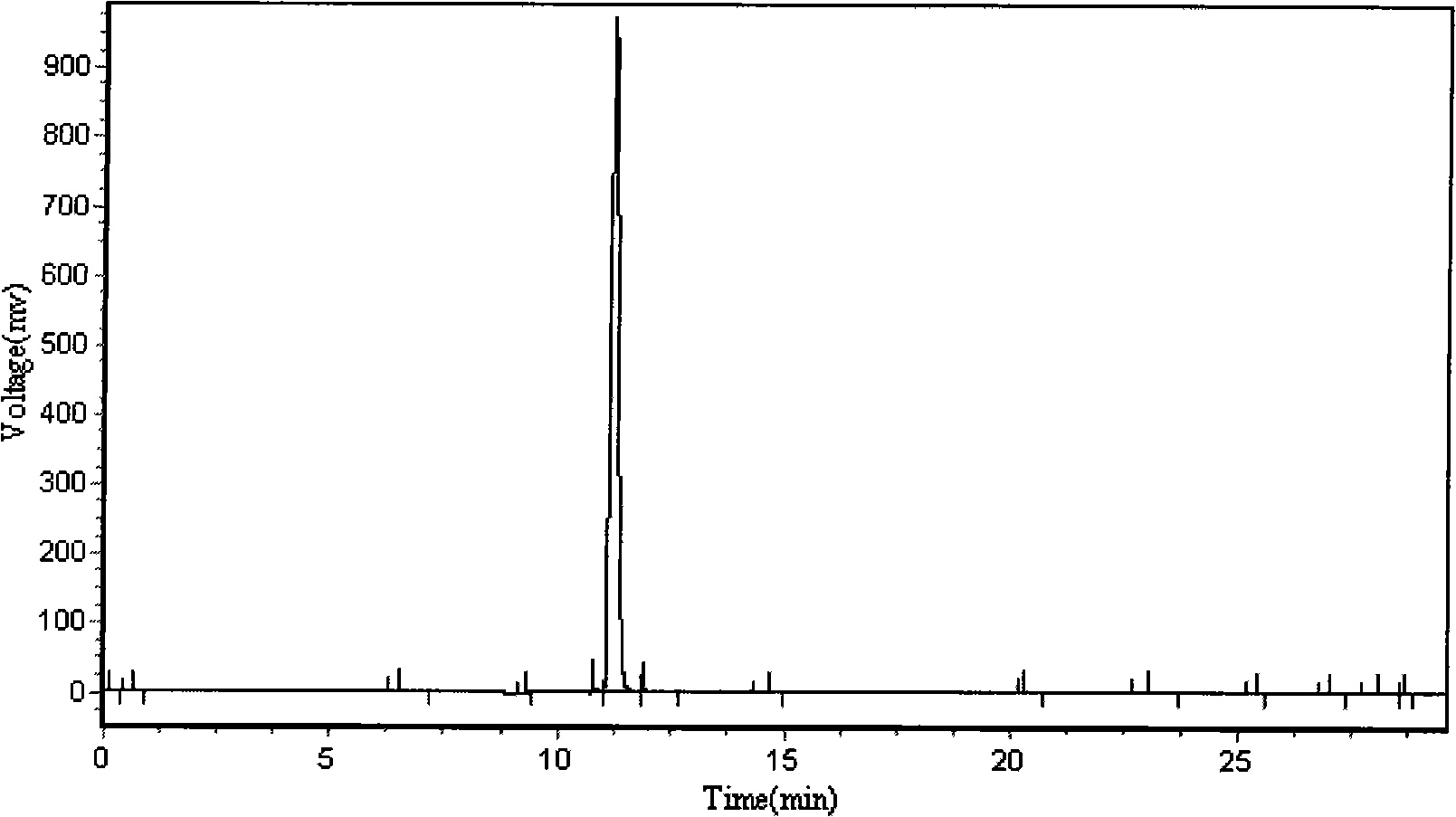
[0053] The self-assembled short peptide R418 prepared in Example 1 was detected by high performance liquid chromatography (HPLC) (detection conditions: phase A was 5% acetonitrile+0.1% trifluoroacetic acid; phase B was 95% acetonitrile+0.1% Trifluoroacetic acid; the linear gradient is 20 minutes), the test results are shown in figure 1 ,according to figure 1 The peak area in the spectrum confirmed that its purity reached 95%.
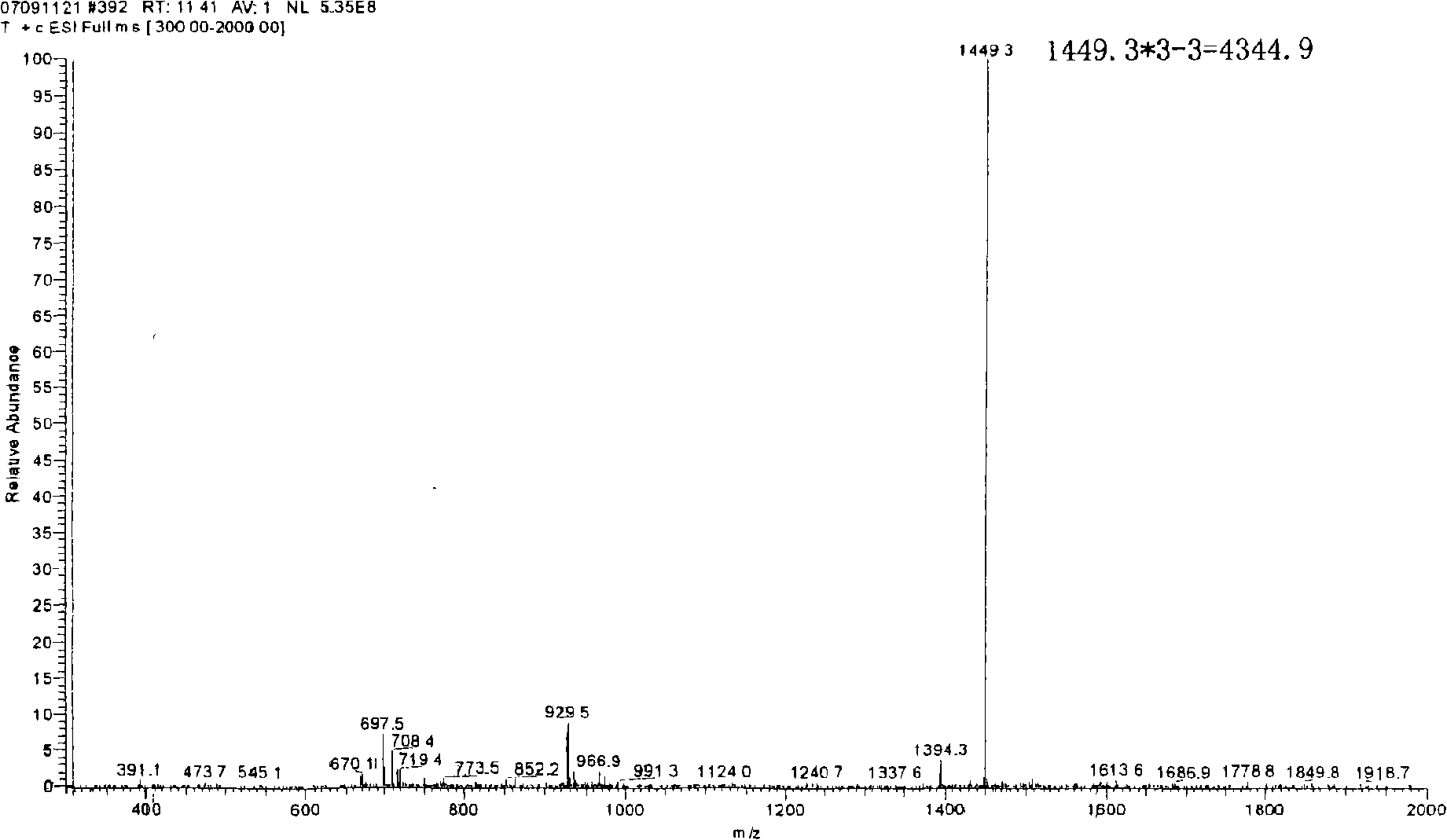
[0054] The self-assembled short peptide R418 prepared in Example 1 is detected by mass spectrometry (the mass spectrometer is Finnigan LCQ), and the detection results are shown in figure 2 ,according to figure 2 It can be determined that its molecular weight is 4344.9 (theoretical molecular weight is 4344.11), indicating that the synthesized short peptide is indeed the designed peptide.
Embodiment 3
[0055] Example 3: Three-dimensional molecular model and self-assembly model drawing of R418 anti-tumor self-assembly short peptide
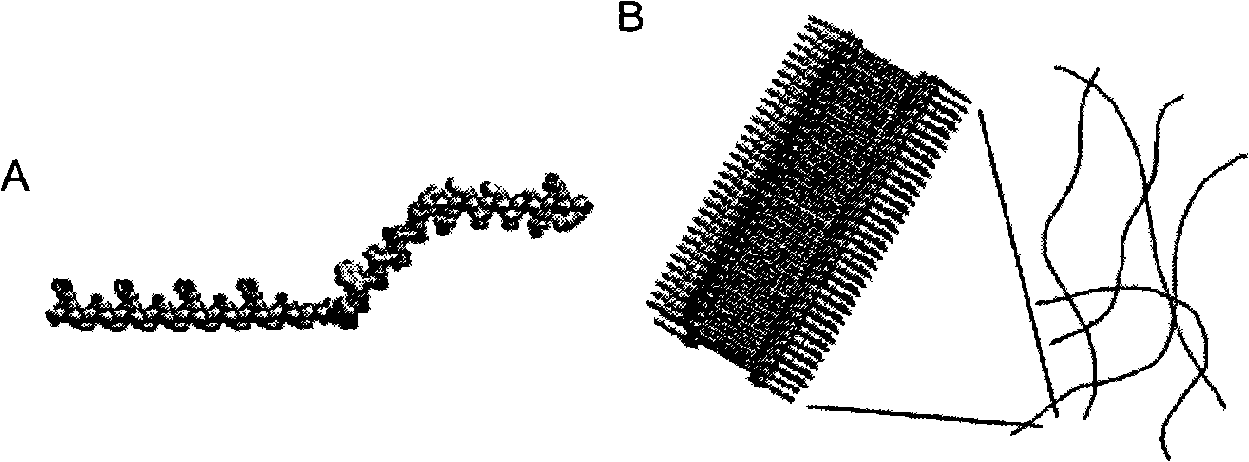
[0056] For the self-assembled short peptide R418 prepared in Example 1, the professional Molsoft.ICM drawing software is used to draw a schematic diagram of a three-dimensional molecular model based on the principle of energy minimization. The schematic diagram of the drawn three-dimensional molecular model is shown in image 3 A, the spatial distribution of its amino acids can be clearly known through the schematic diagram, which shows that the anti-tumor active peptide S18 is located at the C-terminus of the self-assembling peptide RADA16-I, and the two are connected by a linker.
[0057] The self-assembled short peptide R418 prepared in Example 1 can form self-assembled nanofibers in water, and the self-assembled nanofibers formed by the self-assembled short peptide R418 are drawn using professional Molsoft.ICM drawing software based on the prin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average weight | aaaaa | aaaaa |
| Average weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com