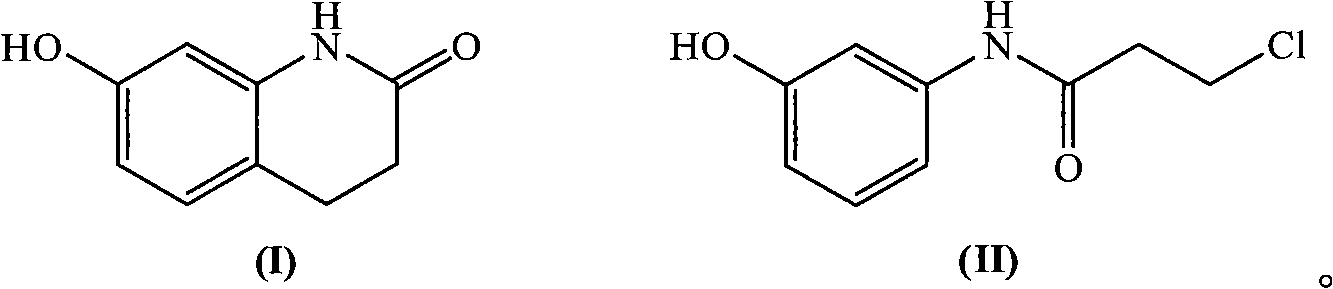
Novel synthetic method of 7-hydroxy-3,4-dihydroquinolines
A technique for the synthesis of dihydroquinolones and methods, which is applied in the field of new synthesis of 7-hydroxy-3,4-dihydroquinolones, which can solve the problems of low reaction yield and large pollution, and achieve good selectivity and low discharge of three wastes , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
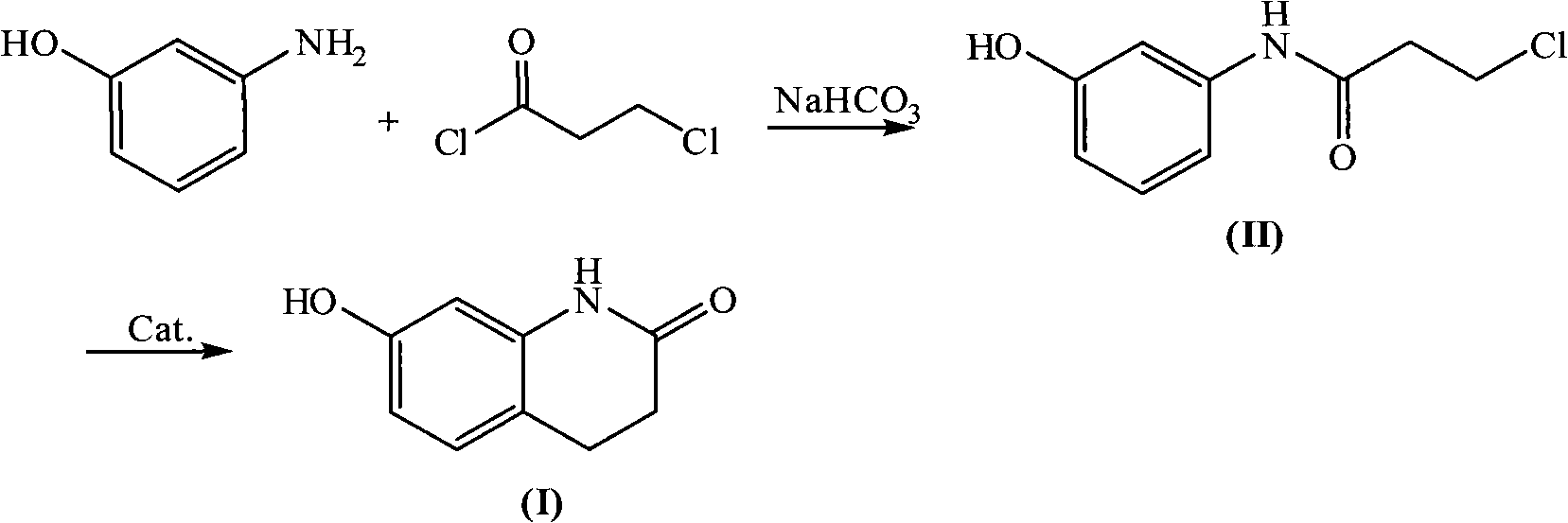
[0026] Embodiment 1: Synthesis of N-(3-chloropropionyl)-3-hydroxyaniline
[0027] Add 25.0g (0.229mol) of 3-hydroxyaniline, 19.3g (0.230mol) of sodium bicarbonate, 0.25g of tetrabutylammonium chloride and 200ml of water into a 500ml three-necked flask, stir and cool the reaction solution with ice water to 5 ~10°C, slowly add 29.3g (0.230mol) 3-chloropropionyl chloride dropwise, after the dropwise addition, keep it warm at 5~10°C for about 1.5 hours, filter under reduced pressure, wash the filter cake with water, and then evaporate to dryness 41.2 g of white solid, namely N-(3-chloropropionyl)-3-hydroxyaniline, was obtained with a yield of 90.2%, mp: 132°C, and a purity of 99.4% by HPLC.
Embodiment 2
[0028] Embodiment 2: Synthesis of 7-hydroxyl-3,4-dihydroquinolones
[0029] Put 15.0g (0.075mol) of N-(3-chloropropionyl)-3-hydroxyaniline and 15.0g of HZSM-5 molecular sieve into a reaction flask, heat up to melt the reactants, and keep stirring at about 160°C for about 1 hour , TLC tracking reaction is complete, after cooling slightly, add 300ml of water in the reaction bottle, reflux and stir for about 1 hour, filter while hot to reclaim molecular sieve, the filtrate is cooled and crystallized, and the crystal is recrystallized with 300ml of water to obtain 8.6g of white crystal product. That is, 7-hydroxy-3,4-dihydroquinolone, the yield is 70.3%, mp: 233°C-235°C, and the purity by HPLC is 99.0%.
Embodiment 3
[0030] Embodiment 3: Synthesis of N-(3-chloropropionyl)-3-hydroxyaniline
[0031] Add 32.7g (0.300mol) of 3-hydroxyaniline, 27g (0.321mol) of sodium bicarbonate, 0.33g of tetrabutylammonium chloride and 250ml of water into a 500ml three-necked flask, stir and cool the reaction solution with ice water to 5~ Slowly add 41.9g (0.330mol) of 3-chloropropionyl chloride dropwise at 10°C. After the dropwise addition, keep the reaction at 5-10°C for about 2 hours, filter under reduced pressure, wash the filter cake with water, and then dry it by rotary evaporation. 54.0 g of white solid, namely N-(3-chloropropionyl)-3-hydroxyaniline, was obtained with a yield of 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com