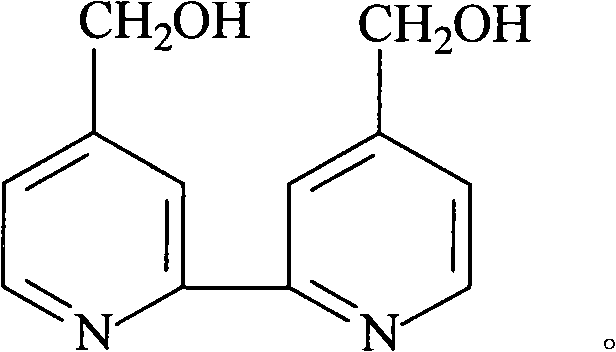
Bidentate ligand 4,4í»- bis (hydroxymethyl)-2,2í»dipyridine, preparation thereof and application to polyketone synthesis
A technology of bidentate ligand and dimethylol, which is applied in the field of catalysts for the synthesis of polyketones, can solve the problems of low catalytic activity and relative molecular weight, and achieve high relative molecular weight, good catalytic activity and improved relative molecular weight. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
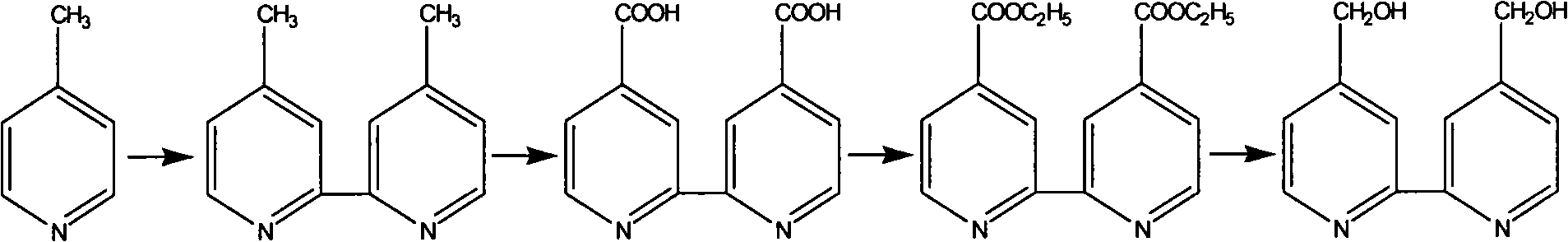
[0026] 4,4'-dimethylol-2,2'-bipyridine synthesis reaction:
[0027]
[0028] Add the distilled 4-picoline and palladium-carbon catalyst in the ratio of 40:1 (g / g) to a 250mL three-necked flask in turn, stir, and heat in an oil bath after mixing evenly. Reflux at 160°C for 72h, stop the reaction, and filter after cooling. The filtrate was collected, and the resulting filtrate was distilled under reduced pressure until a white solid was precipitated. After collecting the solid, recrystallize it with ethyl acetate to obtain 4,4'-dimethyl-2,2'-bipyridyl crystals with a melting point of 171-172°C.
[0029] Under magnetic stirring, 9.7 mmol of Na 2 Cr 2 o 7 Slowly added to concentrated sulfuric acid, and after stirring for 30 min, 4.3 mmol of 4,4'-dimethyl-2,2'-bipyridine was further added thereto. Orange-yellow flocs were formed immediately in the solution, and the stirring was continued, the flocs gradually turned dark green, and the reaction stopped after 30 minutes. The...
Embodiment 2
[0038] Embodiment 2 (for comparison)
[0039] Under the same conditions as in Example 1, a copolymer of carbon monoxide and styrene was prepared. The difference is that the amount of bidentate ligand was changed from 0.1mmol to 0.125mmol to obtain 7.01g of copolymerized product with a catalytic activity of 1318gSTCO / gPd·h. Relative molecular mass M n =8450, M w =13275, M w / M n = 1.572.
Embodiment 3
[0040] Embodiment 3 (for comparison)
[0041] Under the same conditions as in Example 1, a copolymer of carbon monoxide and styrene was prepared. The difference is that the bidentate ligand was changed from 4,4'-dimethylol-2,2'-bipyridine to 2,2'-bipyridine, and 4.41 g of copolymerized product was obtained with a catalytic activity of 829 gSTCO / gPd h . Relative molecular mass M n =4392, Mw=6261, Mw / Mn=1.426.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com