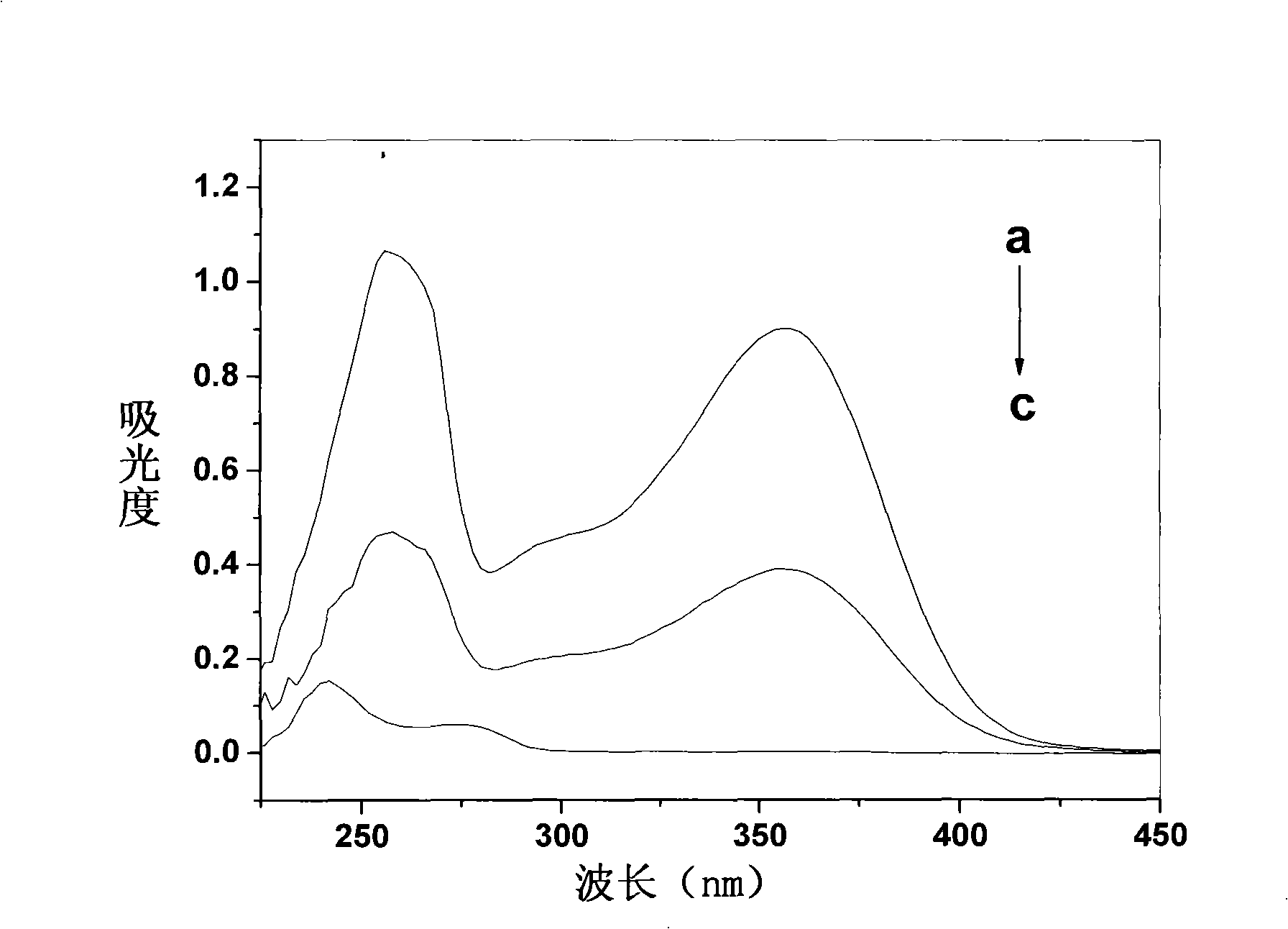
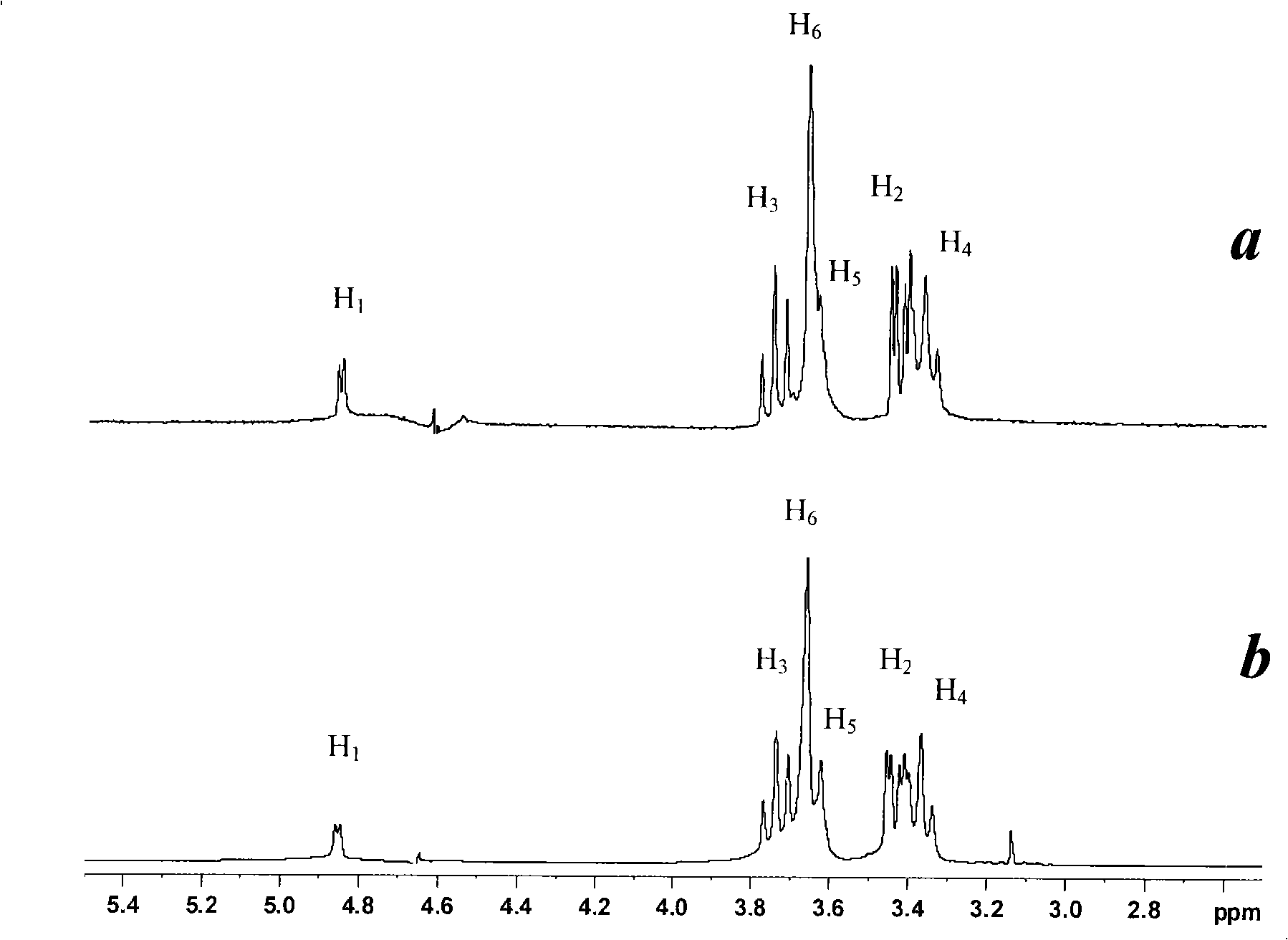
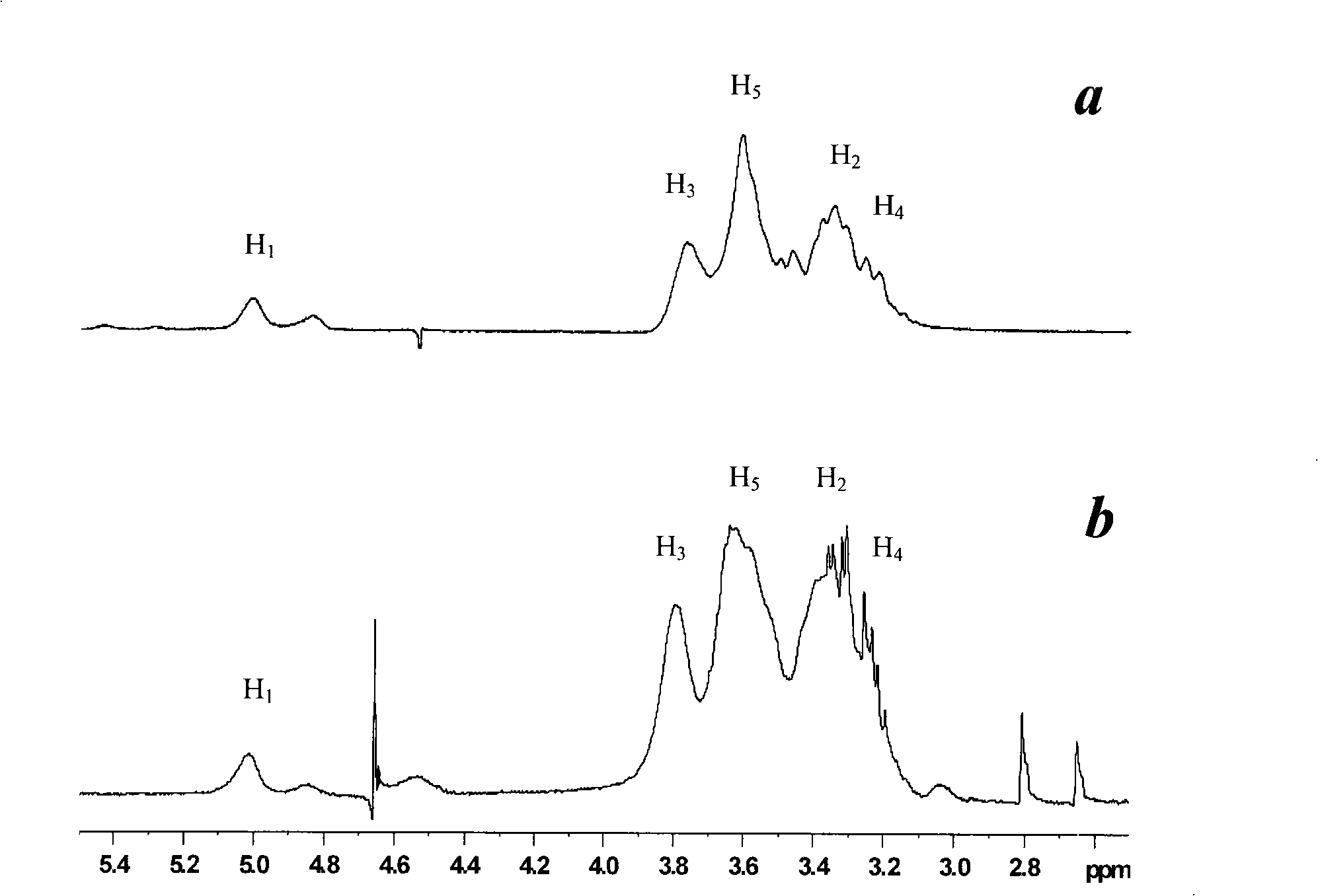
Isoquercitrin clathrate and preparation thereof
A technology of isoquercitrin and inclusion compound, which is applied in the field of isoquercitrin inclusion compound, can solve the problems of good water solubility of the isoquercitrin inclusion compound and the like, and achieves convenient industrial production, high inclusion rate, and preparation. The effect of simple and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Accurately weigh 0.1 g of methyl-β-cyclodextrin and add it to 30 ml of distilled water to dissolve it. Accurately weigh 0.05g of isoquercitrin, add it into 10ml of anhydrous methanol and dissolve it completely, then slowly add it dropwise into the above methyl-β-cyclodextrin aqueous solution under magnetic stirring, and keep stirring at a constant temperature of 50°C for 12 hours , Concentrated, dried at 50°C for 10 hours to obtain the inclusion compound of isoquercitrin-methyl-β-cyclodextrin.
[0028] The content (w / w) of isoquercitrin in the clathrate is 33.78%, the yield is 91.53%, and the encapsulation efficiency is 90.23%.
Embodiment 2
[0029] Example 2: Accurately weigh 0.48 g of β-cyclodextrin, add it to 25 ml of distilled water, and heat to 50° C. to dissolve it. Accurately weigh 0.2 g of isoquercitrin, add it into 10 ml of anhydrous methanol and dissolve it completely, slowly add it dropwise into the above-mentioned β-cyclodextrin aqueous solution under magnetic stirring, keep stirring at constant temperature for 12 hours, and place in the refrigerator for 4 Stand at ℃ for 24 hours, filter with suction, rinse gently with a small amount of methanol solution, and dry the precipitate at 50℃ for 10 hours to obtain the inclusion compound of isoquercitrin-β-cyclodextrin.
[0030] The content of isoquercitrin in the clathrate (w / w)=29.18%, the yield is 75.22%, and the encapsulation efficiency=75.16%.
Embodiment 3
[0031] Example 3: Accurately weigh 0.05 g of hydroxypropyl-β-cyclodextrin and add it to 30 ml of distilled water to dissolve it. Accurately weigh 0.185g of isoquercitrin, add it into 10ml of anhydrous methanol and dissolve it completely, then slowly add it dropwise into the above-mentioned aqueous solution of hydroxypropyl-β-cyclodextrin under ultrasonication, and continue ultrasonication at 50°C for 4 hours, After concentration, dry at 50°C for 10 hours to obtain the inclusion compound of isoquercitrin-hydroxypropyl-β-cyclodextrin.
[0032] The content (w / w) of isoquercitrin in the clathrate is 20.97%, the yield is 92.10%, and the encapsulation efficiency is 90.07%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com