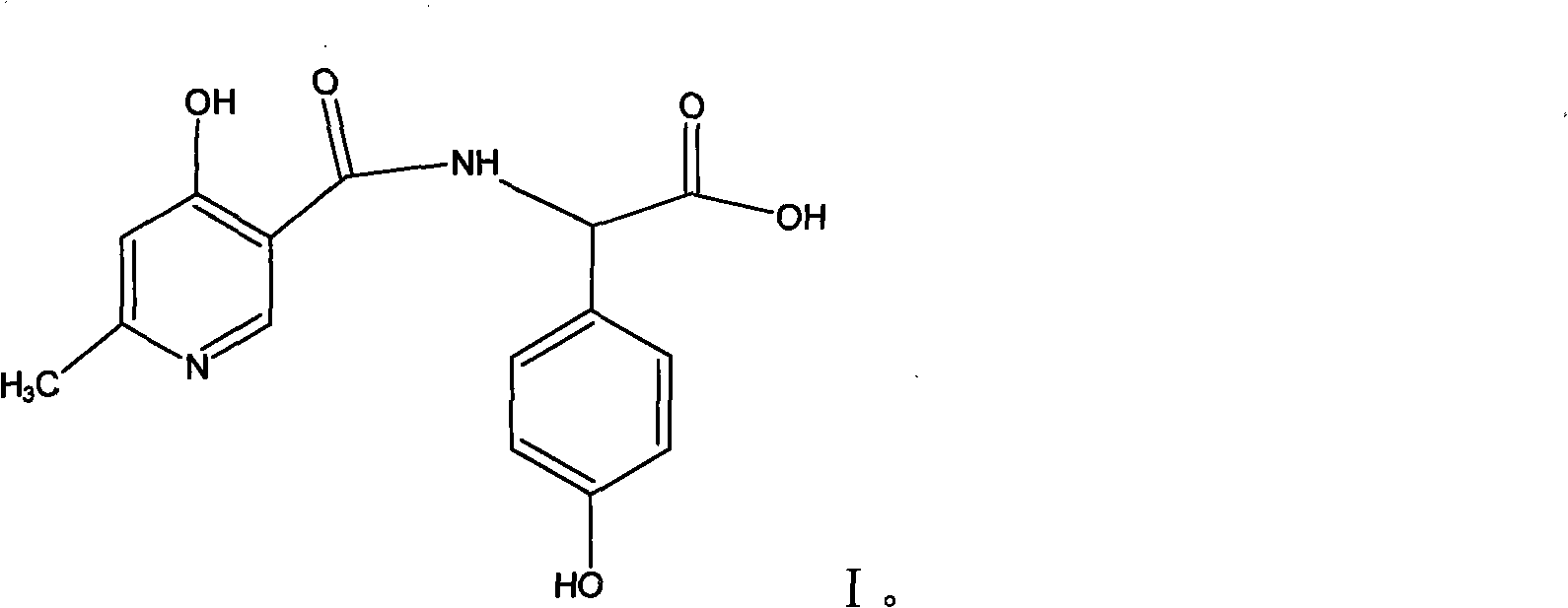
Process for synthesizing D-alpha-(6-methyl-4-hydroxyl nicotinamide base)p-hydroxyphenylacetic acid
A technology based on hydroxynicotinamide and p-hydroxyphenyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of high equipment and operation requirements, harsh reaction conditions, long synthesis routes, etc., and achieve easy control of process parameters, strong Competitive advantage, the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
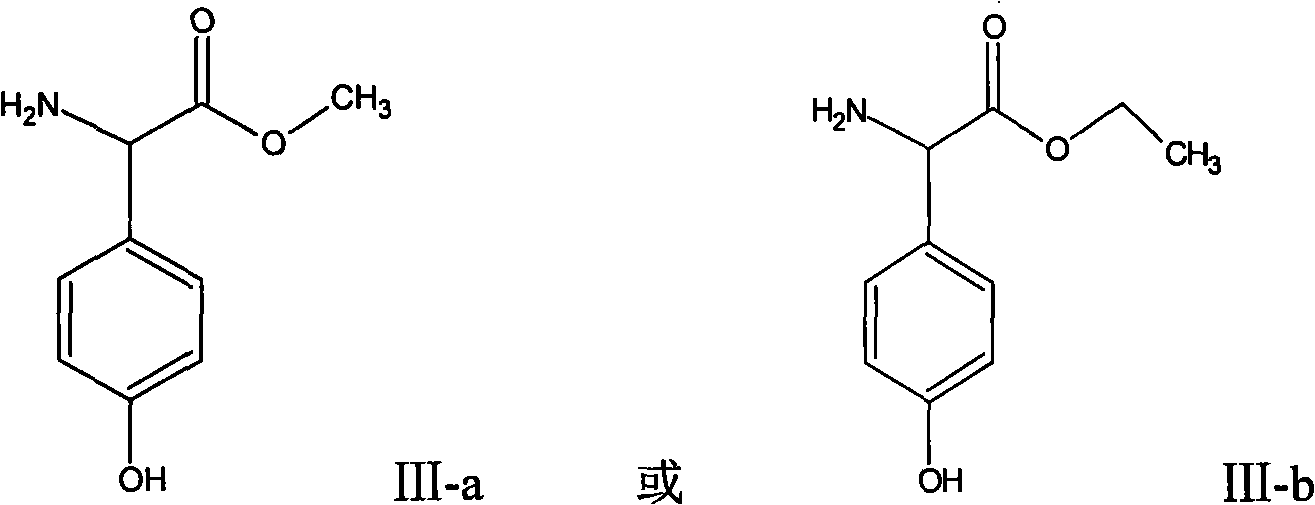
[0030] Synthesis (III-a) of embodiment 1.D-p-hydroxyphenylglycine methyl ester
[0031] Add 180g of D-p-hydroxyphenylglycine to 600ml of methanol, add 100ml of concentrated sulfuric acid under rapid stirring, keep warm at 40°C-50°C for 2 hours, slowly transfer the reaction solution into 1200ml of ice water, keep warm at 0°C to 5°C, The pH was adjusted to neutral with ammonia water, the white crystals were filtered out, and washed with 300 ml of purified water to obtain 171 g of D-p-hydroxyphenylglycine methyl ester.
Embodiment 2
[0032] The synthesis (III-b) of embodiment 2.D-p-hydroxyphenylglycine ethyl ester
[0033] Add 150g of D-p-hydroxyphenylglycine to 600ml of ethanol, add 100ml of concentrated sulfuric acid under rapid stirring, keep warm at 40°C-50°C for 3 hours, transfer the reaction solution slowly into 1200ml of ice water, keep warm at 0°C to 5°C, The pH was adjusted to neutral with ammonia water, the white crystals were filtered out, and washed with 300 ml of purified water to obtain 175 g of D-p-hydroxyphenylglycine ethyl ester.
Embodiment 3
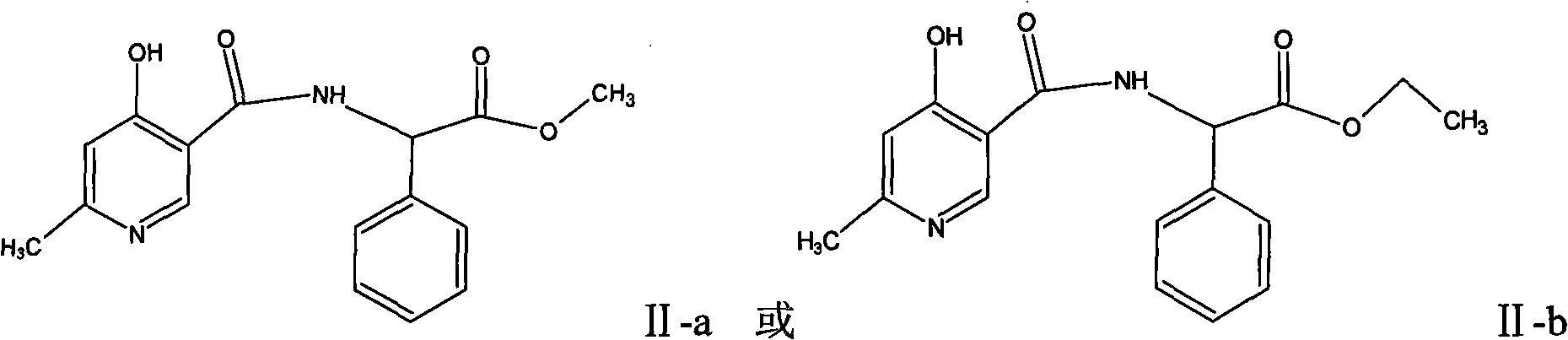
[0034] Example 3.D-α-(6-methyl-4-hydroxynicotinamide base) synthesis of methyl p-hydroxyphenylacetate (II-a)
[0035] 122.5 g of 4-hydroxy-6-methylnicotinic acid were put into 600 ml of dichloromethane. Add 97ml of triethylamine dropwise, drop the temperature at -35°C, add 114g of thionyl chloride dropwise, keep it at -25°C to -20°C for 1 hour, add 160g of D-hydroxyphenylglycine methyl ester, keep it at -25°C to -20°C ℃ for 2 hours, and the temperature was raised to 0 ℃ to continue the reaction for 2 hours. Add 400 ml of purified water and stir rapidly for 3 hours, filter out the solid, wash with 200 ml of purified water, and dry in vacuo to obtain 209 g of methyl D-α-(6-methyl-4-hydroxynicotinamide) p-hydroxyphenylacetate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com