Electrolyte and method for electrolytic deposition of gold-copper alloys
A gold-copper alloy, electrolytic deposition technology, applied in the field of electrolytic composition, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
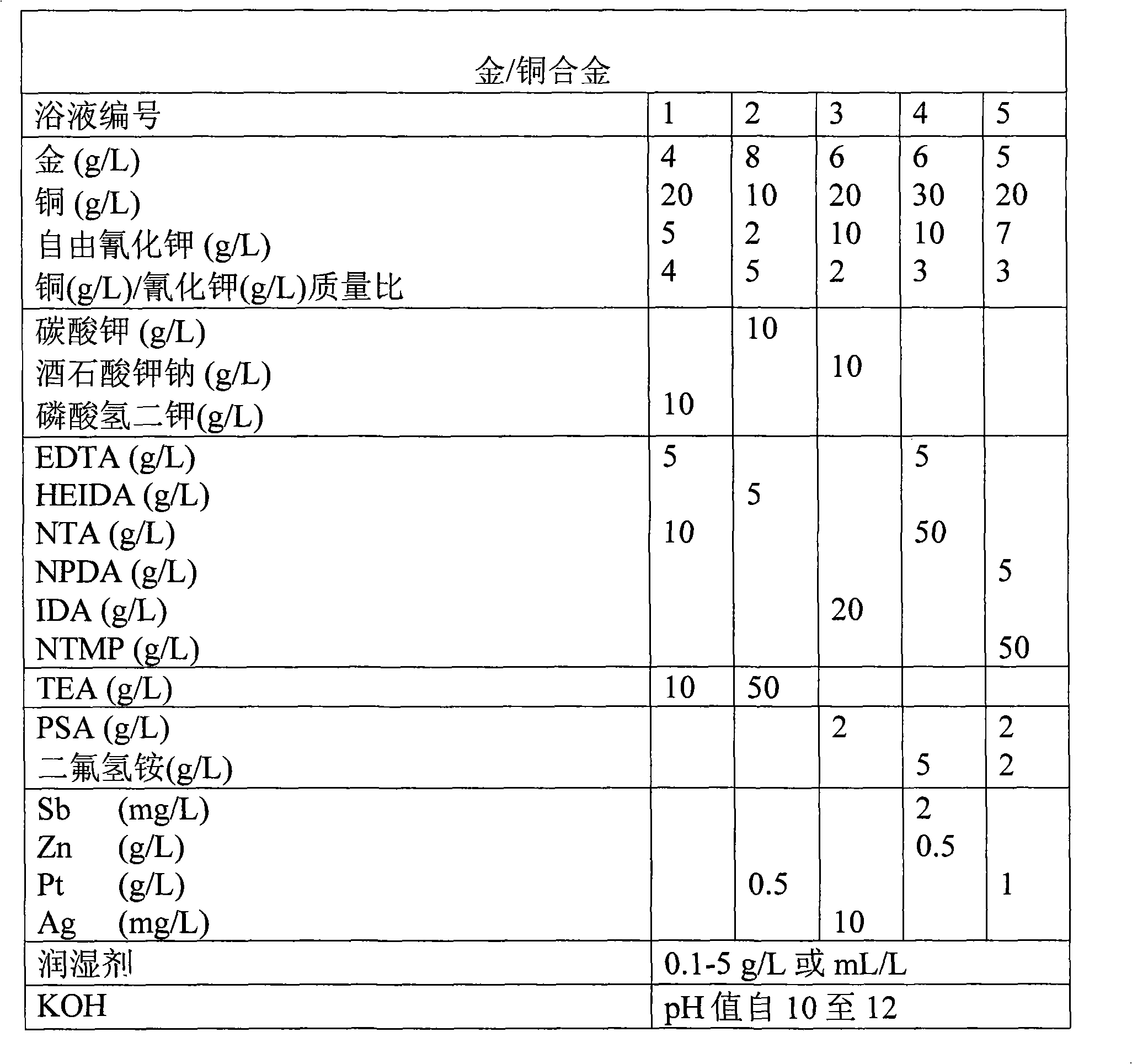
[0011] The present invention relates to an electrolytic composition for electrolytic deposition of a gold-copper alloy onto the surface of a substrate, wherein the electrolytic composition comprises a source of gold ions, a source of copper ions, potassium cyanide and at least one complexing agent, the The complexing agent is selected from the group consisting of ethylenediaminetetraacetic acid [EDTA], diethylenetriaminepentaacetic acid and nitrilotriacetic acid [NTA], hydroxyethyliminodiacetic acid [HEIDA], nitrilopropionic acid Diacetic acid [NPDA], iminodiacetic acid [IDA], nitrilotrimethylphosphoric acid [NTMA, Dequest 2000], triethanolamine [TEA], where the concentration of copper ions and potassium cyanide (KCN) make copper / free potassium cyanide mass ratio in the range of 3-7.
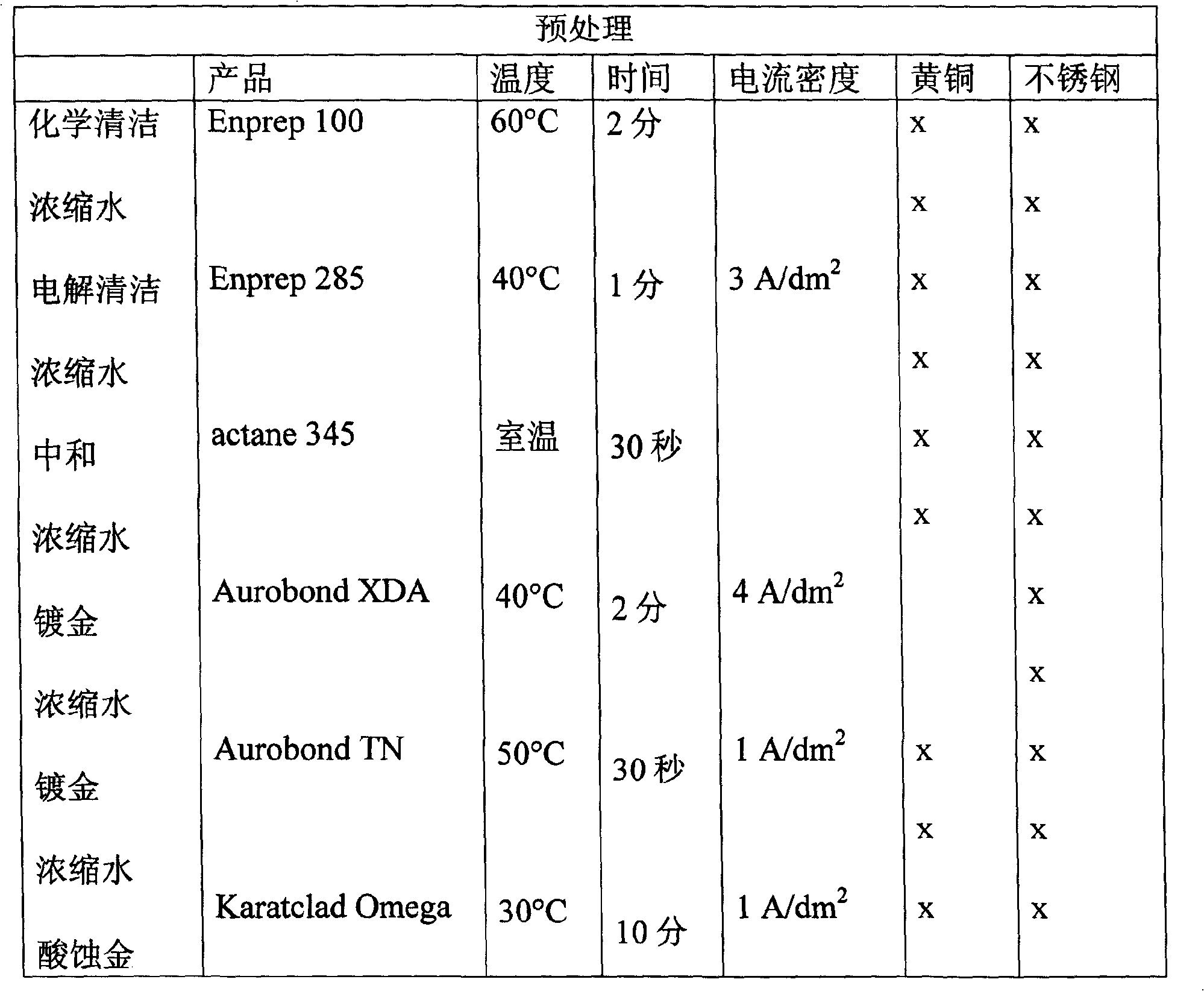
[0012] The present invention further relates to a method of electrolytically depositing gold-copper alloys using the composition described above, whereby the carat value and color of the depos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com