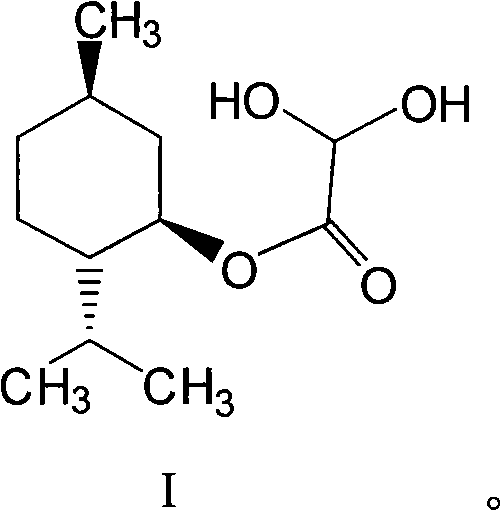
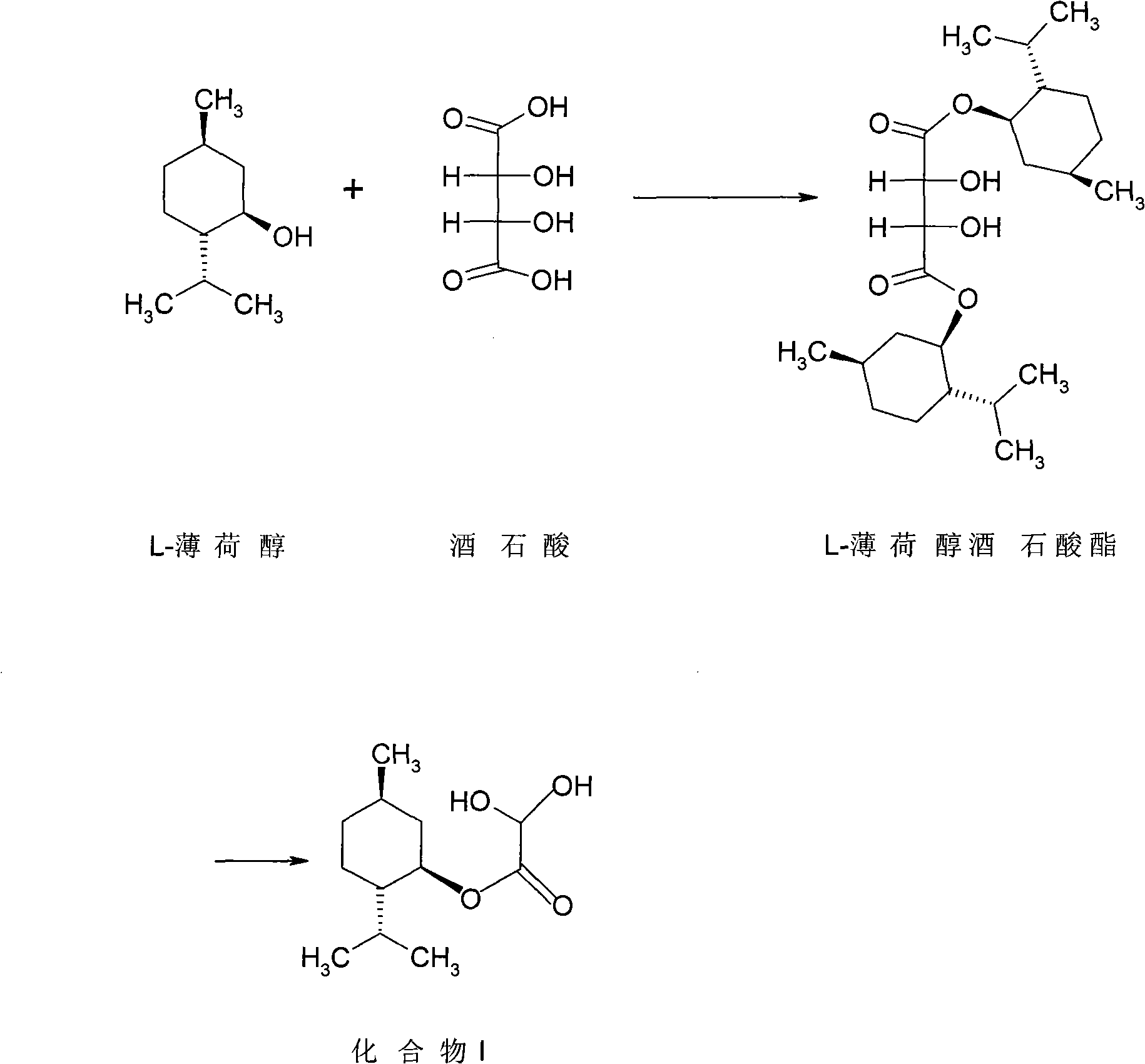
Method for preparing L-menthol glyoxylic ester monohydrate with solid acid as catalyst
A technology of menthol glyoxylate and monohydrate is applied in the field of preparation of L-menthol glyoxylate monohydrate to achieve the effects of reducing waste acid discharge, high yield and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: SO 4 2- / TiO 2 Preparation of L-Menthol Glyoxylate Monohydrate Catalyzed by Solid Superacid
[0029] Step 1: In a 250mL three-necked flask equipped with a stirrer and a thermometer, add 50g of cyclohexane and 57g of L-menthol, and stir at about 25°C until completely dissolved. Add 0.9 g of SO 4 2- / TiO 2 Solid super acid, slowly add 17.6g glyoxylic acid (50% aqueous solution) dropwise under stirring, the temperature is controlled at 22-26°C, and the addition is completed within half an hour. After the addition, install the upper water separator and condenser tube, stir and heat the reactant to slowly raise the temperature, and reflux for 5 hours. The water taken out is separated by the water separator, and the quality of the water is recorded.
[0030] Step 2: After the reaction, the SO 4 2- / TiO 2 Filter the solid superacid, rinse with cyclohexane, combine the organic layers, adjust the pH to about 5.0 with 10% sodium hydroxide, add sodium bisulfit...
Embodiment 2
[0033] Embodiment 2: reclaim SO 4 2- / TiO 2 Preparation of L-Menthol Glyoxylate Monohydrate Catalyzed by Solid Superacid
[0034] With the 0.78g SO recovered above 4 2- / TiO 2 Solid superacid plus 0.12 g new SO 4 2 - / TiO 2 The solid superacid is used as the catalyst, and the others are operated according to Example 1 to obtain the product: 22.6 grams, the yield: 80.7%, and the GC content: 99.1%.
Embodiment 3
[0035] Embodiment 3: Sodium bisulfate catalyzes preparation of L-menthol glyoxylate monohydrate
[0036] Replace SO with 0.6 g of sodium bisulfate 4 2- / TiO 2 Solid superacid, other operation according to Example 1, product: 21.8 grams, yield: 77.8%, GC content: 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com