Quality control method of capsules for curing waist pain
A quality control method and capsule technology, which can be used in capsule delivery, medical preparations containing active ingredients, and measuring devices, etc. It can solve the problem that Yaoxitong capsules should not be taken in large quantities, taken for a long time, microscopic features cannot be seen, and the index components are black. There are no suitable detection methods for Taurine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
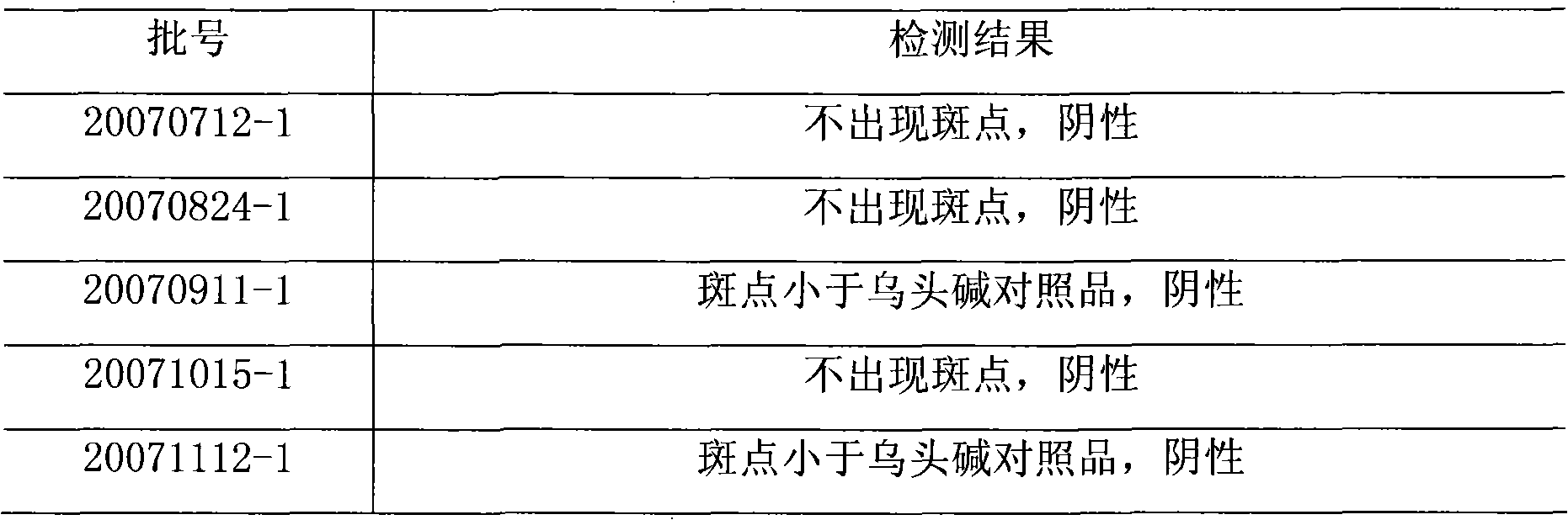
[0044] Five batches of Yaoxitong capsules were randomly selected (batch numbers: 20070712-1, 20070824-1, 20070911-1, 20071015-1, 20071112-1).
[0045] Take 6.0g of the contents of the above 5 batches of capsules, put them in a 500ml Erlenmeyer flask with a stopper, add 60ml of concentrated ammonia test solution to make it wet, add 200ml of ether, ultrasonicate for 40 minutes, let cool, and divide the ether solution , Evaporate to dryness, add absolute ethanol to the residue to dissolve and dilute to 2 ml, as the test solution. In addition, accurately weigh an appropriate amount of aconitine reference substance, add absolute ethanol to make a solution containing 1 mg per 1 ml, and use it as the reference substance solution. Test according to the thin-layer chromatography (Appendix VIB of the Chinese Pharmacopoeia in 2005), draw each 5 microliters of the above-mentioned need testing solution and the reference solution, and point respectively on the same silica gel G thin film wi...
experiment example 2
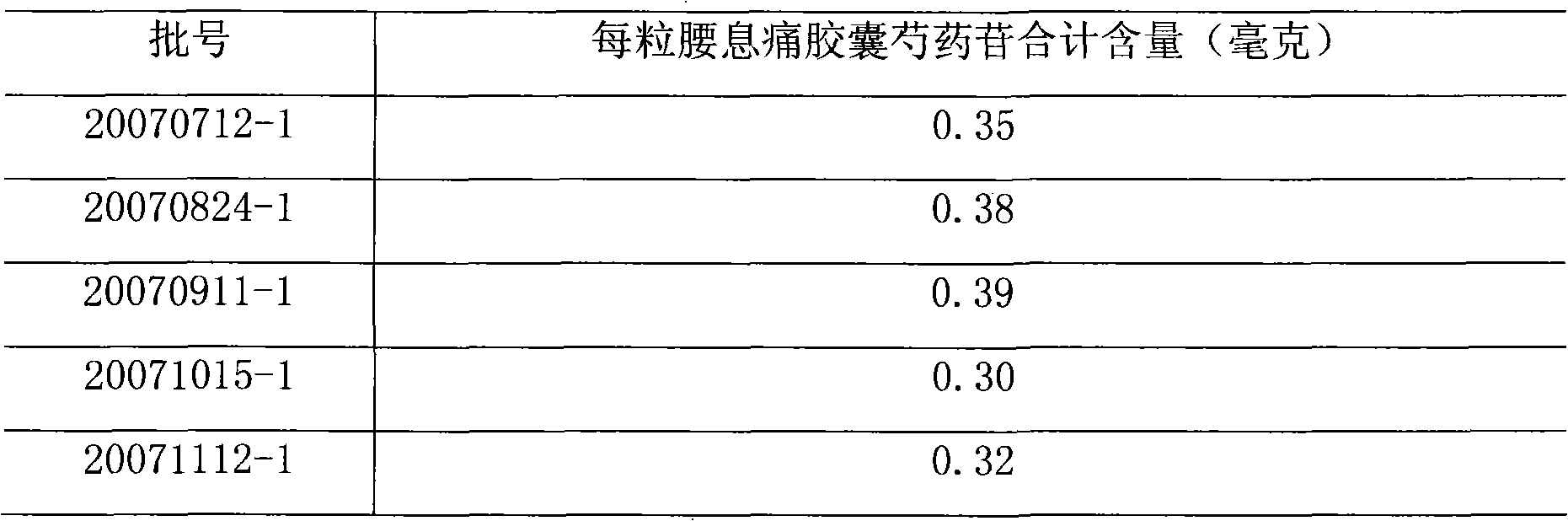
[0050] Five batches of Yaoxitong capsules were randomly selected (batch numbers: 20070712-1, 20070824-1, 20070911-1, 20071015-1, 20071112-1).
[0051] Determination according to high performance liquid chromatography (Chinese Pharmacopoeia 2005 edition appendix VID).
[0052] Chromatographic conditions and system suitability test use octadecylsilane bonded silica gel as filler, acetonitrile-0.05% phosphoric acid aqueous solution (14:86) as mobile phase, detection wavelength 230nm, flow rate: 1.0 ml / min, theoretical plate number press Paeoniflorin peak calculation should not be less than 3000.
[0053] Preparation of reference substance solution Take an appropriate amount of paeoniflorin reference substance dried under reduced pressure to constant weight, accurately weigh it, add 50% methanol to make a solution containing 60 micrograms per 1 ml, and obtain it.
[0054] Preparation of the test solution Take an appropriate amount of the content of the Yaoxitong Capsules under th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com