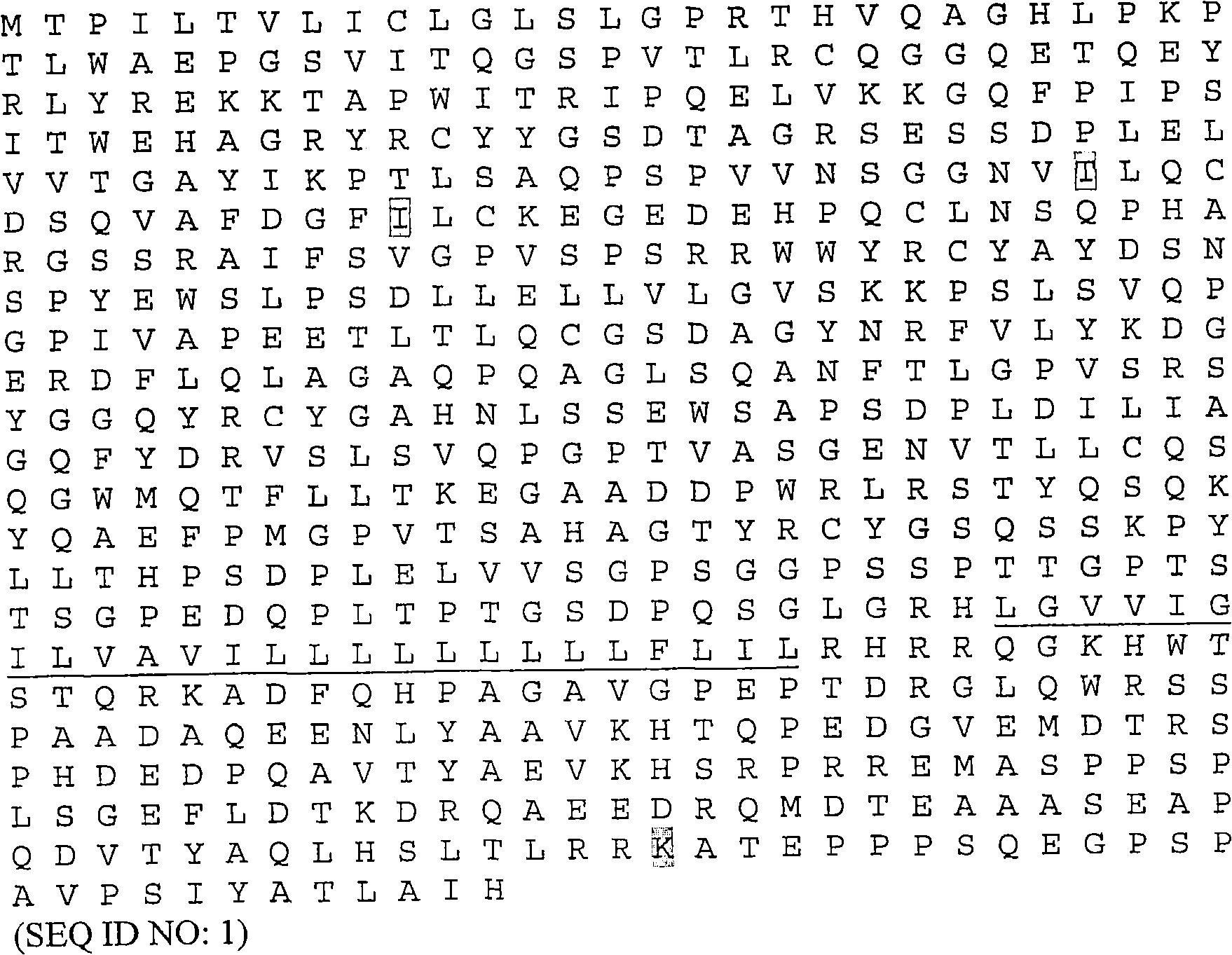
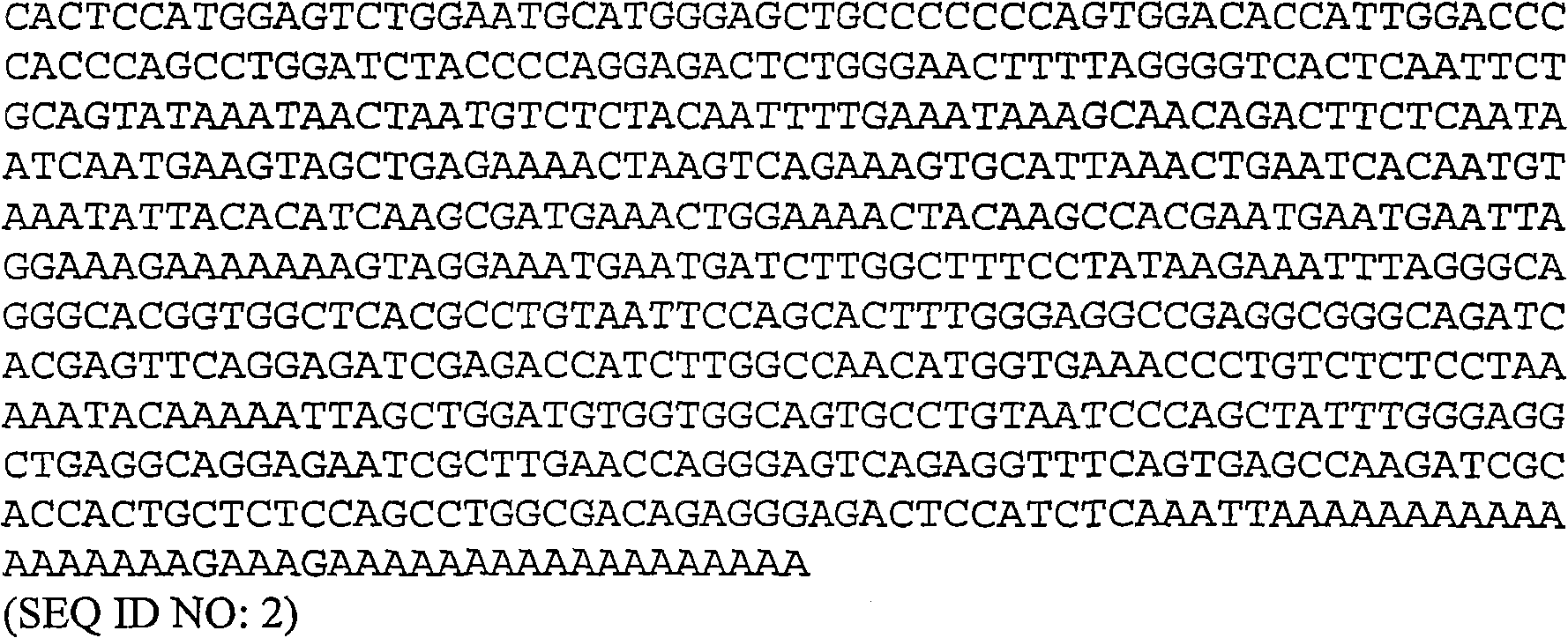Modified leukocyte ig-like receptor family members (LIR'S) with increased affinity for class I MHC and their uses in modulating T cell activation
A cell-use technology, applied in the field of modified leukocyte Ig-like receptor family (LIR'S) with improved affinity for class I MHC and its use in regulating T cell activity, can solve problems such as slow affinity dissociation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1 - Preparation of Soluble Wild-Type ILT-2 Molecules Containing Domains 1 and 2
[0155] image 3 (SEQ ID NO: 5) provides the DNA sequence used to express soluble wild-type ILT-2 containing only domains D1 and D2. These DNA sequences can be synthesized de novo by various contract research companies, eg GeneArt (Germany). Restriction enzyme recognition sites (NdeI and HindIII) were introduced into this DNA sequence, thereby facilitating the ligation of these DNA sequences into a pGMT7-based expression plasmid containing the gene for high-level expression in E. coli strain BL21-DE3 (pLysS). Expressed T7 promoter (Pan et al., Biotechniques (2000) 29(6):1234-8).
[0156] The DNA sequence is connected into the pGMT7 carrier cut with NdeI and HindIII (the DNA sequence of the carrier is shown in Figure 6 , see the plasmid map of this vector Figure 7 ).
[0157] Introduction of a restriction enzyme recognition site into DNA encoding a soluble wild-type ILT-2 poly...
Embodiment 2
[0164] Example 2 - Preparation of High Affinity Variants of Soluble Wild-Type ILT-2 Polypeptides
[0165] Soluble wild-type ILT-2 polypeptides produced as described in Example 1 can be used as templates from which polypeptides of the invention with increased affinity and / or reduced off-rates for class I pMHC molecules can be generated.
[0166] It is known to those skilled in the art that the necessary codon changes required to generate these mutant chains can be introduced into the DNA encoding the soluble wild-type ILT-2 polypeptide by site-directed mutagenesis (QuickChange TM , Stratagene Site-Directed Mutagenesis Kit).
[0167] Briefly, this is achieved by using primers incorporating the desired codon change(s) and a plasmid containing DNA encoding a soluble wild-type ILT-2 polypeptide as a template for mutagenesis:
[0168] The following conditions were used for mutagenesis: 50ng of plasmid template, 1μl of 10mM dNTP, 5μl of 10×Pfu DNA polymerase buffer provided by the m...
Embodiment 3
[0170] Example 3 - Expression, refolding and purification of soluble polypeptides
[0171] The expression plasmids containing the ILT polypeptide (gene) prepared in Example 1 or 2 were respectively transformed into Escherichia coli strain rosetta DE3pLysS, and cultured in TYP medium (containing 100 μg / ml ampicillin and 15 μg / ml chloramphenicol) at 37°C Single colonies with ampicillin / chloramphenicol resistance were incubated for 7 hours before protein expression was induced with 0.5 mM IPTG. After 15 hours of induction, the cells were collected by centrifugation at 4000 rpm for 30 minutes with a Beckman J-6B. The cell pellet was resuspended in buffer, and the resuspended cells were sonicated for 1 min with a standard 12 mm diameter probe in a Milsonix XL2020 sonicator for a total of about 10 min. The inclusion body precipitate was recovered by centrifugation at 4000 rpm for 10 minutes with a Beckman J2-21 centrifuge. It was then washed three times with detergent to remove ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com