Expression of human cardiac muscle type fatty acid binding proteins in bacillus coli DH5 alpha and purification
A fatty acid combination and Escherichia coli technology, applied in the biological field, can solve the problems of high price and low yield, and achieve the effect of avoiding denaturation and refolding process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] 1. Plasmids and strains
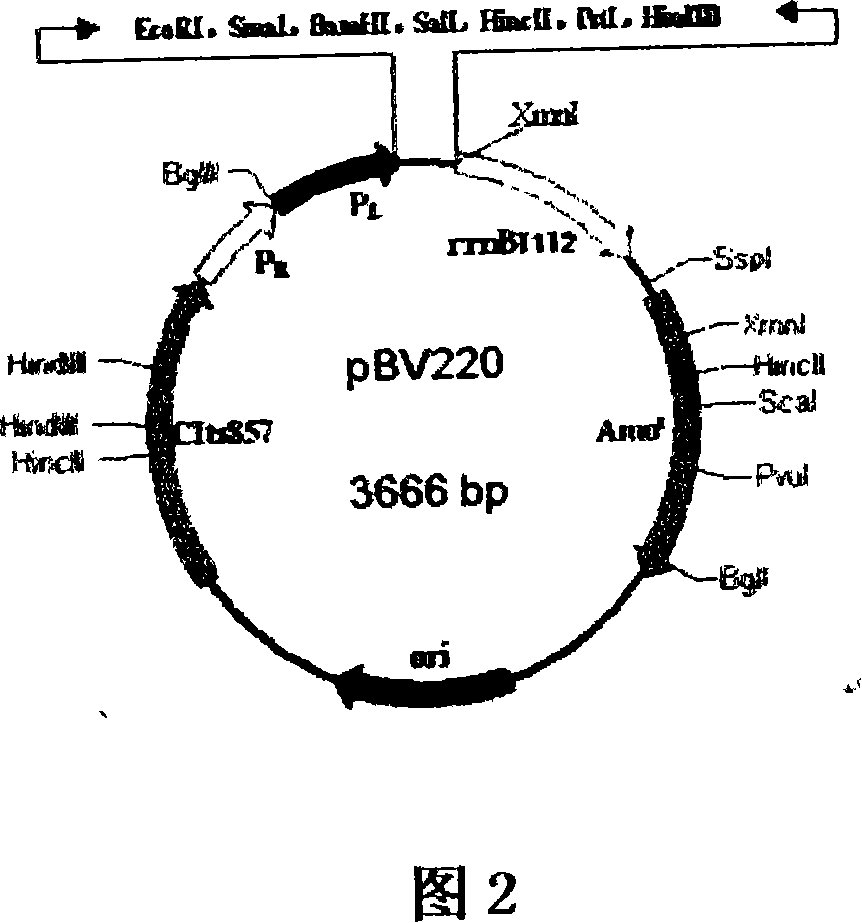
[0097] Escherichia coli (E.coli) DH5α strain, pGEM-T easy, PBV220 protein expression plasmid.
[0098] 2. Tool enzymes and biochemical reagents
[0099] Restriction endonuclease, T4 DNA ligase, Taq DNA polymerase, plasmid DNA extraction kit, DNA gel recovery kit, human H-FABP monoclonal antibody (Clone 6B6), horseradish peroxidase-labeled di Anti, RNAase inhibitor, AMV reverse transcriptase, Sephacryl S-100 HR, Sepharose G-75.
[0100] 3. Oligonucleotide primers
[0101] Following the principles of PCR primer design, according to the human H-FABP cDNA sequence of GenBank (NM004012), the 5' and 3' end oligonucleotide primers encoding the H-FABP gene fragment were designed, and EcoR was introduced at the respective 5' ends. I and Sal I restriction enzyme sites. The primer sequences are as follows:
[0102] Forward primer: 5'-gcg aat tca tgg tgg acg ctt tc-3';
[0103] Reverse primer: 5'-att gtc gac tca tgc ctc ttt ctc-3',
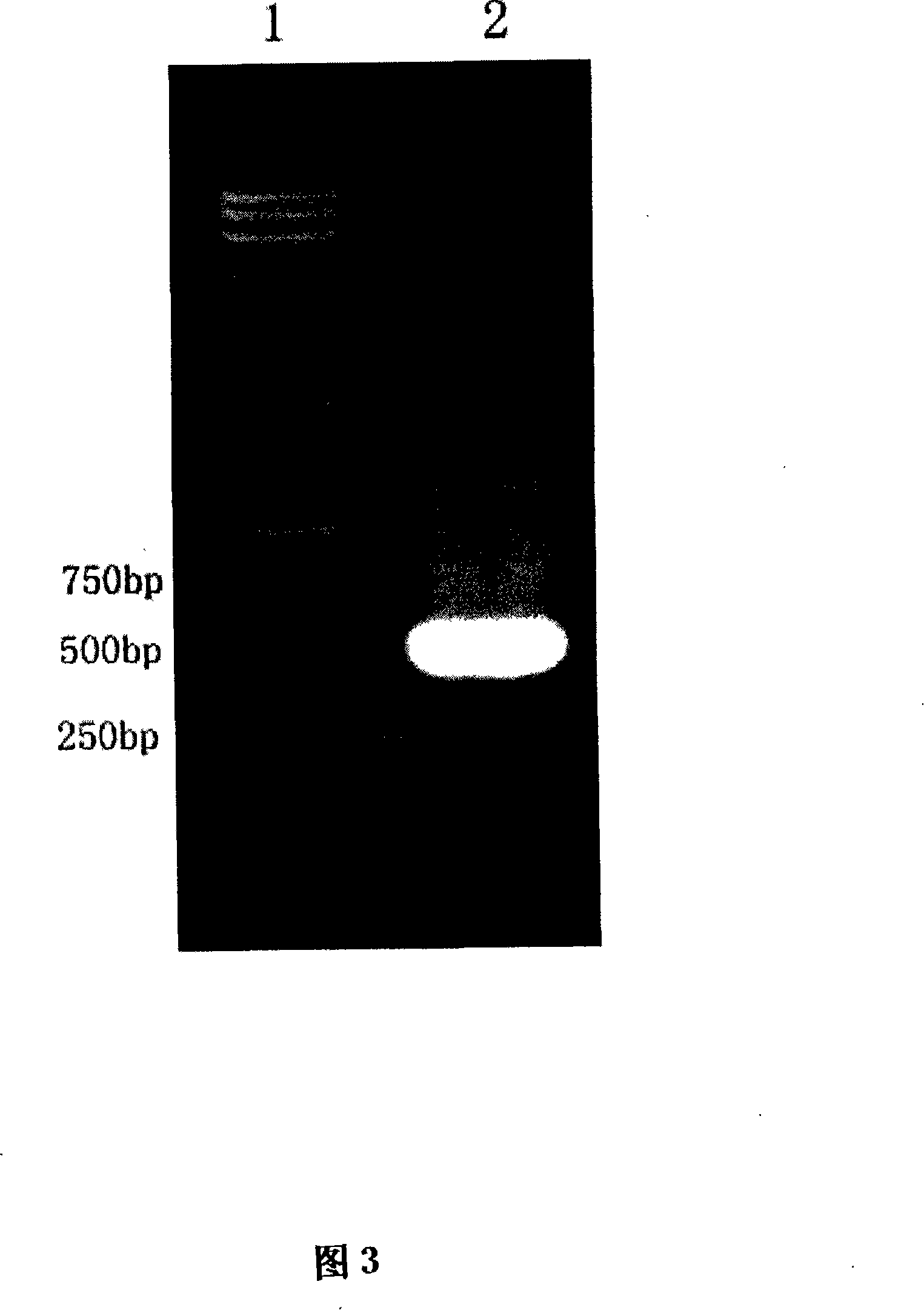
[0104] 4. RT-PC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com