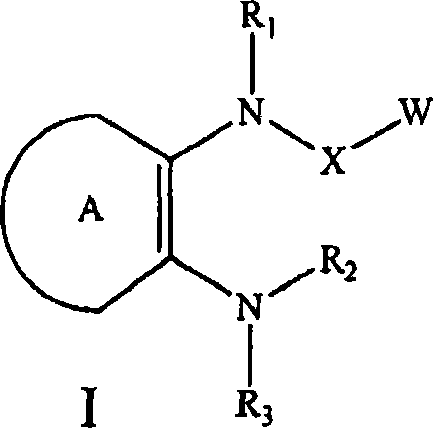
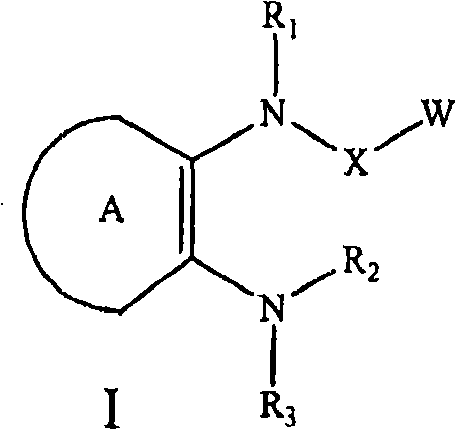
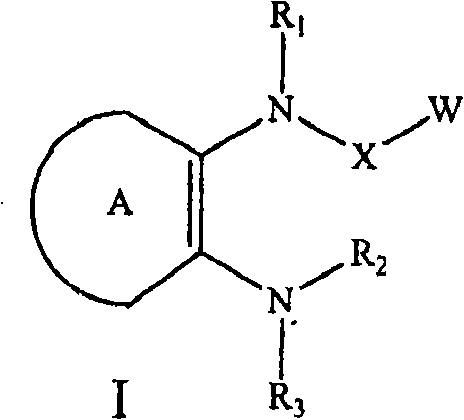
C-fms kinase inhibitors
A technology of solvates and compounds, applied in the field of novel compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] 5-Cyano-furan-2-carboxylic acid [2-(4-acetylamino-piperidin-1-yl)-phenyl]-amide
[0094]
[0095] a) 5-cyano-furan-2-carboxylic acid
[0096]
[0097] To 2-formyl-5-furancarboxylic acid (2.8 g, 20 mmol) and hydroxylamine hydrochloride (2.7 g, 40 mmol) was added anhydrous pyridine (50 mL) under argon. The mixture was heated to 85°C, acetic anhydride (40 mL) was added, and the mixture was stirred for 3 hours. After cooling to 60°C, water (250 mL) was added, and the mixture was stirred at room temperature for 70 hours. The mixture was acidified to pH 2 with concentrated hydrochloric acid and extracted with 3:1 dichloromethane-isopropanol (8 x 100 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over anhydrous sodium sulfate and concentrated in vacuo to give the title compound (1.26 g, 46%) as a tan solid.
[0098] 1 H-NMR (CD 3 OD; 400MHz): δ14.05(br s, 1H), 7.74(d, 1H, J=3.8Hz), 7.42(d, 1H, J=3.8Hz).
[0099] b) 4-acetyla...
Embodiment 2
[0115] 5-Cyano-1H-pyrrole-2-carboxylic acid (2-piperidin-1-yl-phenyl)-amide
[0116]
[0117] a) Ethyl 5-formyl-1H-pyrrole-2-carboxylate
[0118]
[0119] Phosphorus oxychloride (1.5 mL) was added dropwise in DMF (1.3 mL, 17 mmol) cooled to 5-10 °C, and the mixture was diluted with 1,2-dichloroethane (5 mL). The flask was then cooled to -10 °C, 1,2-dichloroethane (5 mL) containing ethyl pyrrole-2-carboxylate (2.00 g, 14.4 mmol) was added dropwise over 5 minutes, and the mixture was heated to reflux for 15 minutes, cooled to room temperature, and added ethyl acetate (15 mL), water (20 mL) and saturated aqueous sodium bicarbonate (70 mL). The layers were separated and the aqueous layer was extracted with diethyl ether (3 x 20 mL). The combined organic layers were washed with saturated sodium carbonate (50 mL), dried over sodium sulfate, the solvent was removed in vacuo and the resulting solid was purified by column chromatography on silica gel (300 g) eluting with 30% et...
Embodiment 3
[0137] 5-Cyano-furan-2-carboxylic acid (4-methoxy-2-piperidin-1-yl-phenyl)-amide
[0138]
[0139] a) 1-(5-Chloro-2-nitro-phenyl)-piperidine
[0140] To a cooled (0° C.) solution of 4-chloro-2-fluoronitrobenzene (1.75 g, 10.0 mmol) in EtOH (15 mL) was added piperidine (2.97 mL, 30.0 mmol) dropwise over 5 minutes. The solution was stirred at 0°C for 10 minutes, then at 23°C for 30 minutes. The mixture was poured into water (225 mL), extracted with EtOAc (2 x 30 mL). The combined extracts were washed with saturated aqueous sodium bicarbonate and brine (30 mL each) and dried (Na 2 SO 4 ). Concentration afforded 2.33 g (97%) of the title compound as an orange oil, which crystallized on standing.
[0141] Mass Spectrum (ESI, m / z): For C 11 h 13 ClN 2 o 2 The calculated value of 241.1 (M+H), the measured value: 241.1.
[0142] b) 1-(5-methoxy-2-nitro-phenyl)-piperidine
[0143] To a solution of 1-(5-chloro-2-nitro-phenyl)-piperidine (197 mg, 0.810 mmol) in DMF (1.5 mL)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com