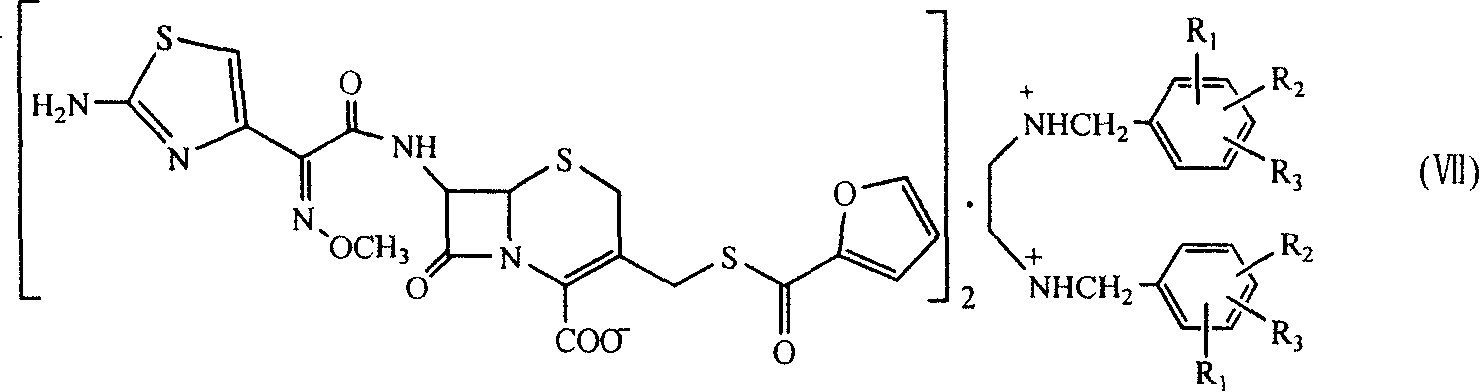
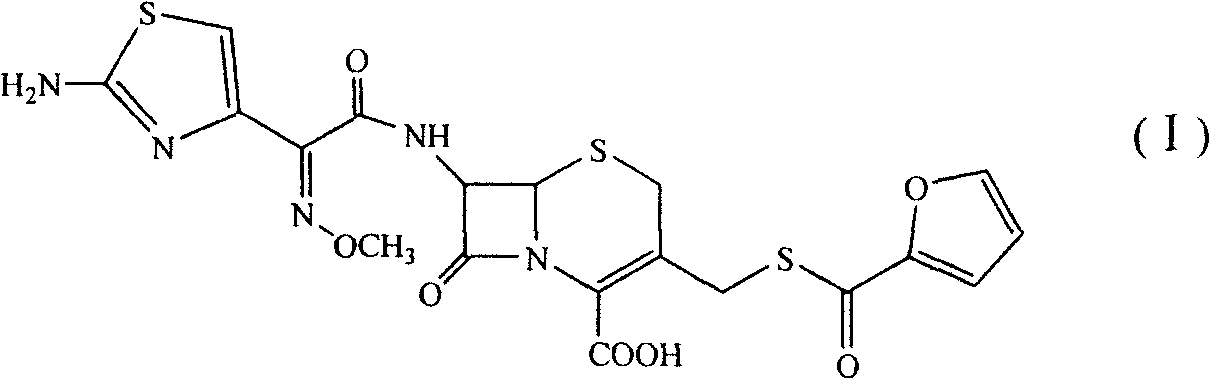
Benzathine salt of ceftiofur, preparation method and application thereof
A ceftiofur and benzyl technology is applied to the application field in the preparation of ceftiofur sodium salt, which can solve the problem of insoluble ceftiofur sodium, purity and solubility of ceftiofur sodium not meeting medicinal requirements, and salt formation of ceftiofur sodium. Incomplete and other problems, to avoid degradation, easy purification, high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of ceftiofur bisbenzylethylenediamine salt
[0043] Cool 200ml of dichloromethane to 0-5°C, add 30.0g of 7-amino-3-[(2-furancarbonyl)thiomethyl]-3-cephem-4-carboxylic acid, 10ml of anhydrous methanol, 20ml of three Ethylamine, then put in 33.0g 2-(2-aminothiazol-4-yl)-(Z)-(methoxyimino)acetic acid benzothiazole thioester (AE active ester), react at 0~5℃ for 8 Hour. Add 100ml of water to the above reaction solution, stir well, let stand, and separate the water phase; then add 100ml of water, stir well, let stand, separate the water phase. The aqueous phases were combined and extracted with 200 ml of dichloromethane. Adjust the pH of the aqueous phase to 6.0-8.0 with dilute hydrochloric acid, add 1 g of activated carbon for decolorization and filtration, wash the activated carbon with 100 ml of water, combine the aqueous phases, add 100 ml of dichloromethane, and stir. Another 20.0 g of bisbenzylethylenediamine diacetate was dissolved in 200 m...
Embodiment 2
[0046] Embodiment 2: Preparation of ceftiofur bisbenzylethylenediamine salt
[0047] The operation is the same as in Example 1, wherein 500 ml of acetone is added to the combined aqueous phase to replace 100 ml of dichloromethane, and 1000 ml of water is added to obtain 47 g of ceftiofur. same.
Embodiment 3
[0048] Embodiment 3: Preparation of ceftiofur bisbenzylethylenediamine salt
[0049] Suspend 60g of ceftiofur hydrochloride in 400ml of water, add dilute ammonia water to adjust the pH to 6.0-8.0, after the solution is clarified, add 2g of activated carbon, stir for 30 minutes, filter, add 200ml of dichloromethane to the filtrate, and stir. Another 28 g of bisbenzylethylenediamine diacetate was dissolved in 300 ml of water. Add the bisbenzylethylenediamine diacetate solution to the above solution to precipitate the bisbenzylethylenediamine salt of ceftiofur, stir evenly, filter, wash with water, and dry in vacuo to obtain 62g of ceftiofur bisbenzylethylenediamine. Amine salt, structure is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com