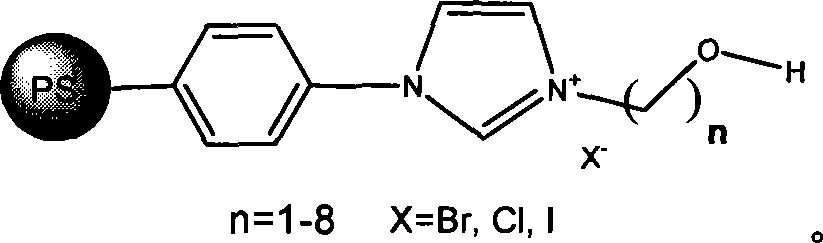
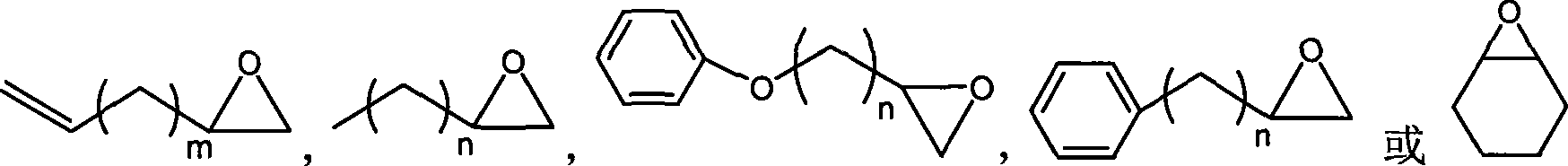
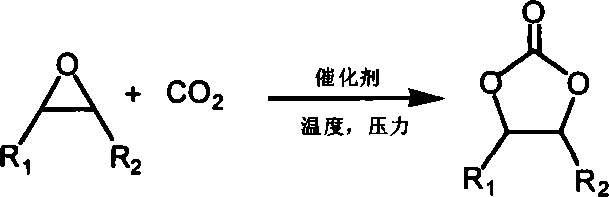
Method for preparing cyclic carbonates from carrying hydroxyl ionic liquid
A cyclic carbonate and ionic liquid technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of active components being sensitive to water, low reactivity, easy Churn and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] Implementation method: in a 100ml stainless steel autoclave, sequentially add 0.5 g of ethanol imidazolium bromide (n=2 in the structural formula) supported by polystyrene resin (D301) (3.0 mmol, 1.5 mol % based on the content of ionic liquid, the following Same), 14ml propylene oxide (1a) (0.2mol), sealed reactor, filled with carbon dioxide of appropriate pressure, controlled temperature by temperature controller to rise slowly to 110 ℃, then controlled reaction pressure to 2.5MPa, reacted for 4.0 hours. After the reaction, the reactor was cooled to 5° C., and excess carbon dioxide was slowly released. After the catalyst was separated by filtration, the obtained product (2a) was analyzed by gas chromatography, and the selectivity was >99.8%, and the yield was 96%.
Embodiment 2
[0022] Same as in Example 1, the catalyst used is 0.4 g (about 3.0 mmol) of ethanol imidazole chloride (n=2 in the structural formula, X=Cl) supported by polystyrene resin, and other conditions are constant, and the selectivity of product (2a) is 98.5 %, the yield is 75%.
Embodiment 3
[0024] Same as Example 1, the catalyst used is 0.5 g (3.2 mmol) of propanol imidazole chloride (n=3 in the structural formula, X=Cl) supported by polystyrene resin (D201), the reaction temperature is 120 ° C, and other conditions are constant , to obtain (2a) with a selectivity of 99% and a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com