Carboxymethyl chitosan quaternary ammonium salt derivatives and preparation method thereof
A technology of carboxymethyl chitosan and quaternary ammonium salt, applied in the field of carboxymethyl chitosan quaternary ammonium salt derivatives and its preparation, to achieve the effects of easy industrialization, overcoming poor solubility, and expanding application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
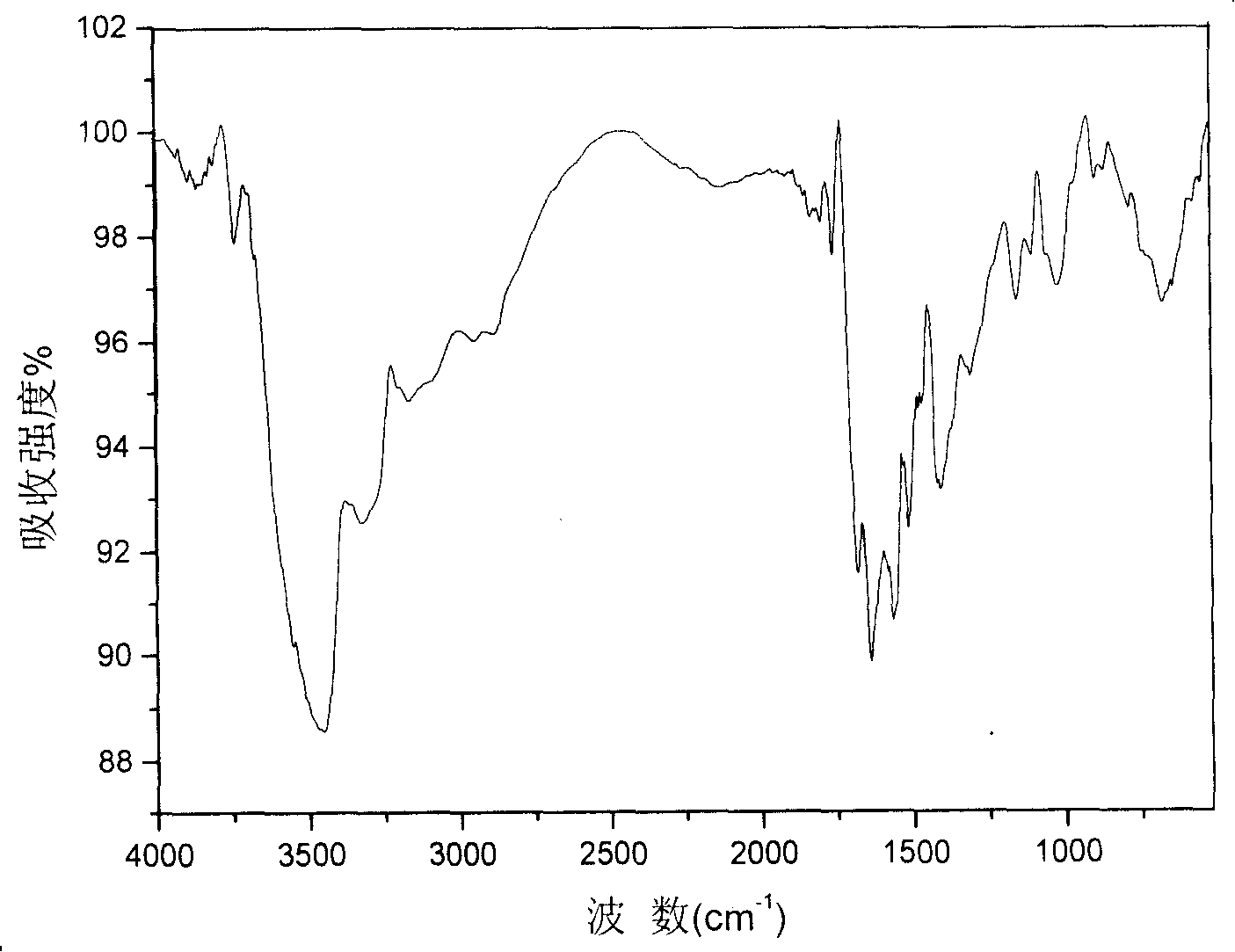
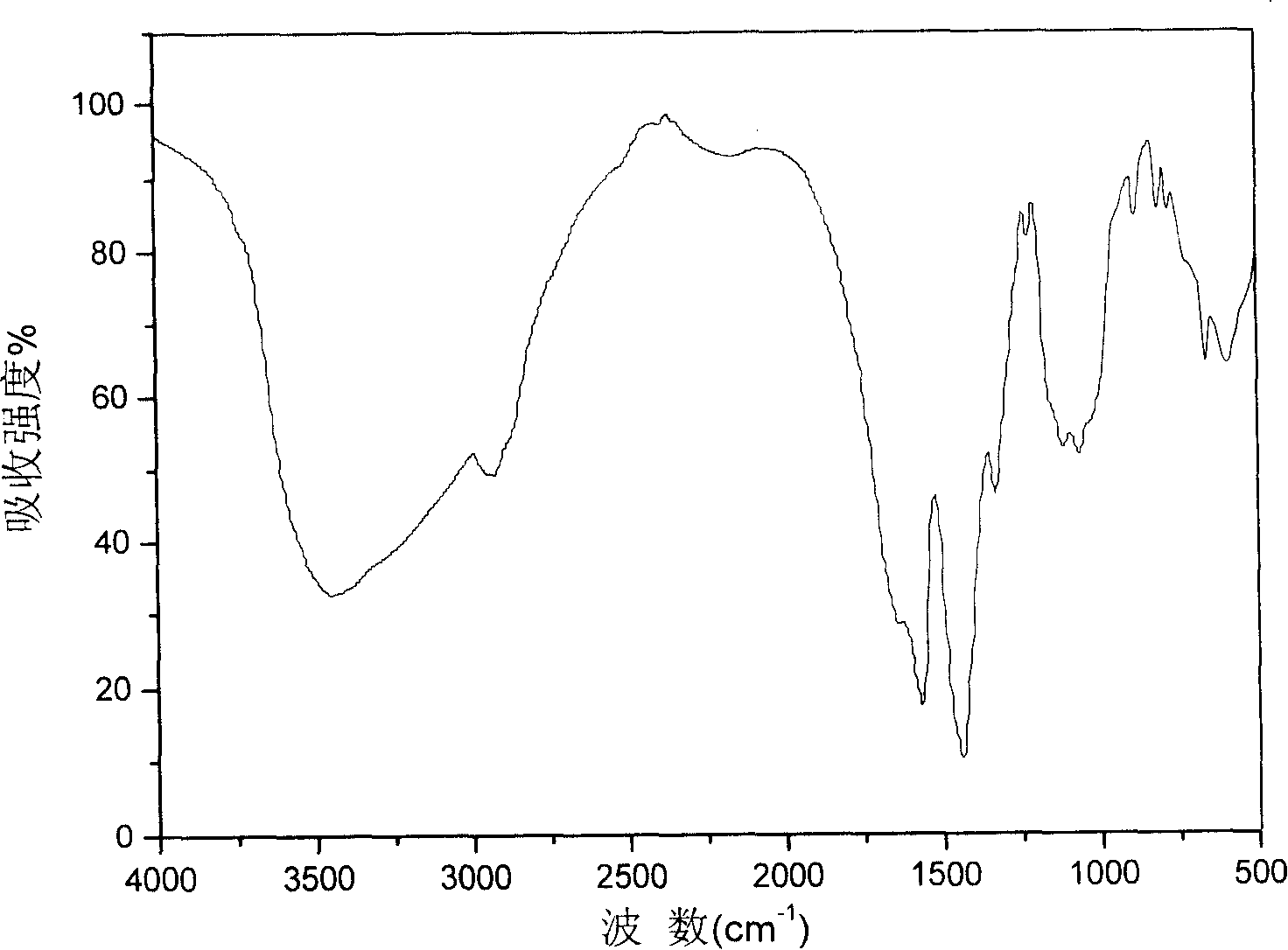
Image
Examples
example 1
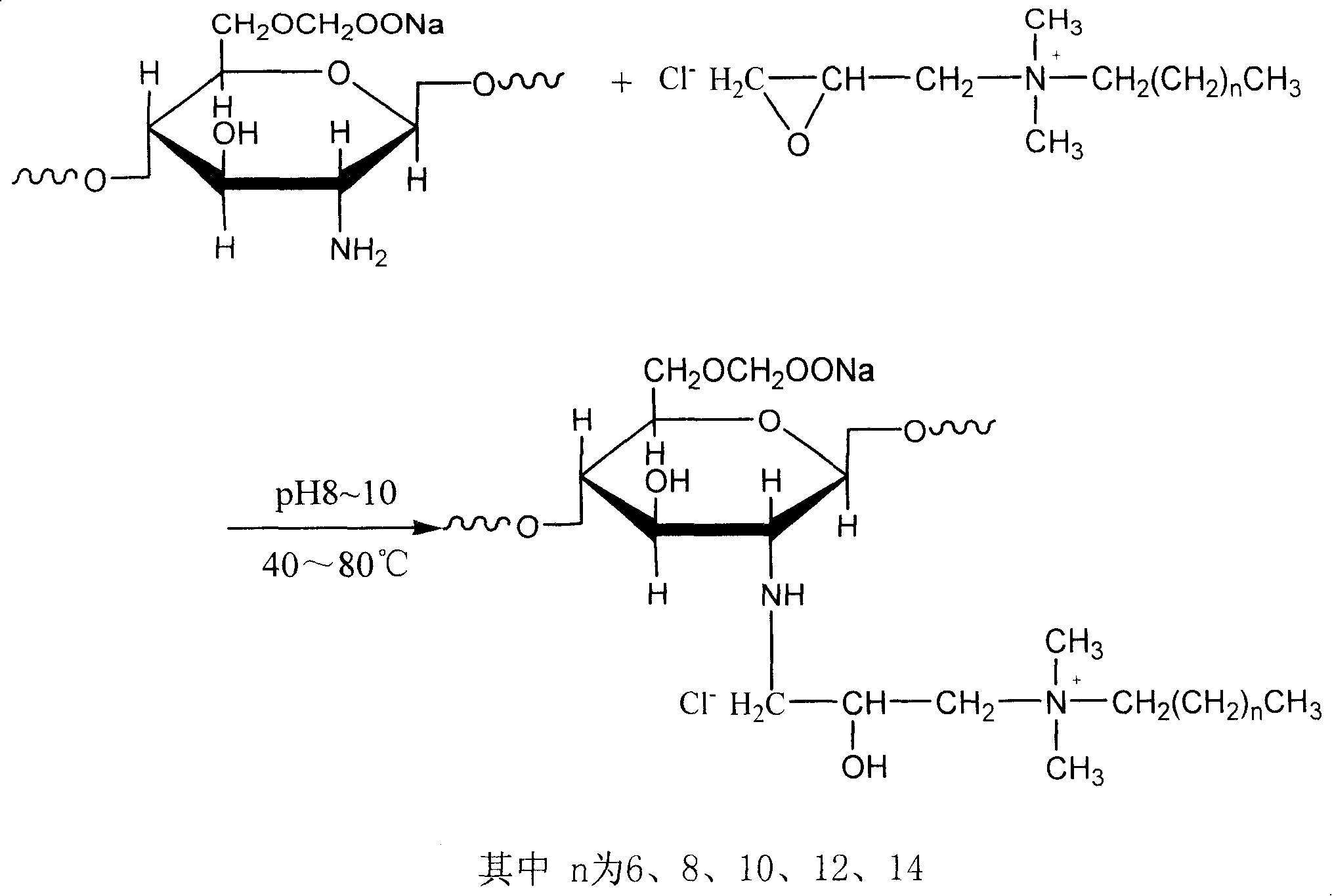
[0036] Synthesis of Epoxypropyl Decyl Dimethyl Ammonium Chloride:
[0037] Add 40ml of isopropanol and 18.5g of dodecyldimethylamine into a 100ml one-necked flask, and heat up to 55°C while stirring. Slowly add 9.3 g of epichlorohydrin into the system dropwise with a constant-pressure dropping funnel. After the dropwise addition, keep the temperature at 55° C. and reflux for 10 hours. After the experiment, distill under reduced pressure to remove unreacted epichlorohydrin and dry to obtain glycidyldecyldimethylammonium chloride.
[0038] Activation of carboxymethyl chitosan:
[0039]Add 25ml of N-methyl-2-pyrrolidone and 0.4g of NaOH powder into a 100ml three-necked flask, stir at 50°C for 1 hour, filter out excess NaOH, and obtain a solvent with a pH of about 9. Add 0.5 g of carboxymethyl chitosan to be activated, and alkalize at 50° C. for 6 hours.
[0040] Quaternization of carboxymethyl chitosan:
[0041] Dissolve 0.56g of glycidyldecyldimethylammonium chloride in 5ml ...
example 2
[0046] Synthesis of epoxypropyl dodecyl dimethyl ammonium chloride:
[0047] Add 40ml of butanone and 21.3g of dodecyldimethylamine into a 100ml one-necked flask, and heat up to 55°C while stirring. Slowly add 9.3 g of epichlorohydrin into the system dropwise with a constant-pressure dropping funnel. After the dropwise addition, keep the temperature at 55° C. and reflux for 10 hours. Distill under reduced pressure after the experiment, remove unreacted epichlorohydrin, and dry to obtain epoxypropyl dodecyl dimethyl ammonium chloride.
[0048] Activation of carboxymethyl chitosan:
[0049] Add 25ml of isopropanol and 0.4g of NaOH powder into a 100ml three-neck flask, stir at 50°C for 1 hour, filter out excess NaOH, and obtain a solvent with a pH of about 9. Add 0.5 g of carboxymethyl chitosan to be activated, and alkalize at 50° C. for 6 hours.
[0050] Quaternization of carboxymethyl chitosan:
[0051] Dissolve 1.22 g of epoxypropyl dodecyl dimethyl ammonium chloride in 5 ...
example 3
[0056] Synthesis of Epoxypropyl Octalkyl Dimethyl Ammonium Chloride:
[0057] Add 40ml of isopropanol and 15.7g of octaalkyldimethylamine into a 100ml one-necked flask, and heat up to 50°C while stirring. Slowly add 9.3 g of epichlorohydrin into the system dropwise with a constant pressure dropping funnel. After the dropwise addition, keep the temperature at 50° C. and reflux for 8 hours. Distill under reduced pressure after the experiment, remove unreacted epichlorohydrin, and dry to obtain epoxypropyl octaalkyl dimethyl ammonium chloride.
[0058] Activation of carboxymethyl chitosan:
[0059] Add 25ml of N-methyl-2-pyrrolidone and 0.4g of NaOH powder into a 100ml three-necked flask, stir at 50°C for 1 hour, filter out excess NaOH, and obtain a solvent with a pH of about 9. Add 0.5 g of carboxymethyl chitosan to be activated, and alkalize at 50° C. for 6 hours.
[0060] Quaternization of carboxymethyl chitosan:
[0061] Dissolve 1.50g of epoxypropyl octaalkyldimethylammo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com