Intravascular deliverable stent for reinforcement of vascular abnormalities
一种血管、畸形的技术,应用在独特支架或移植物领域,能够解决伤害内皮和狭窄损伤部位等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
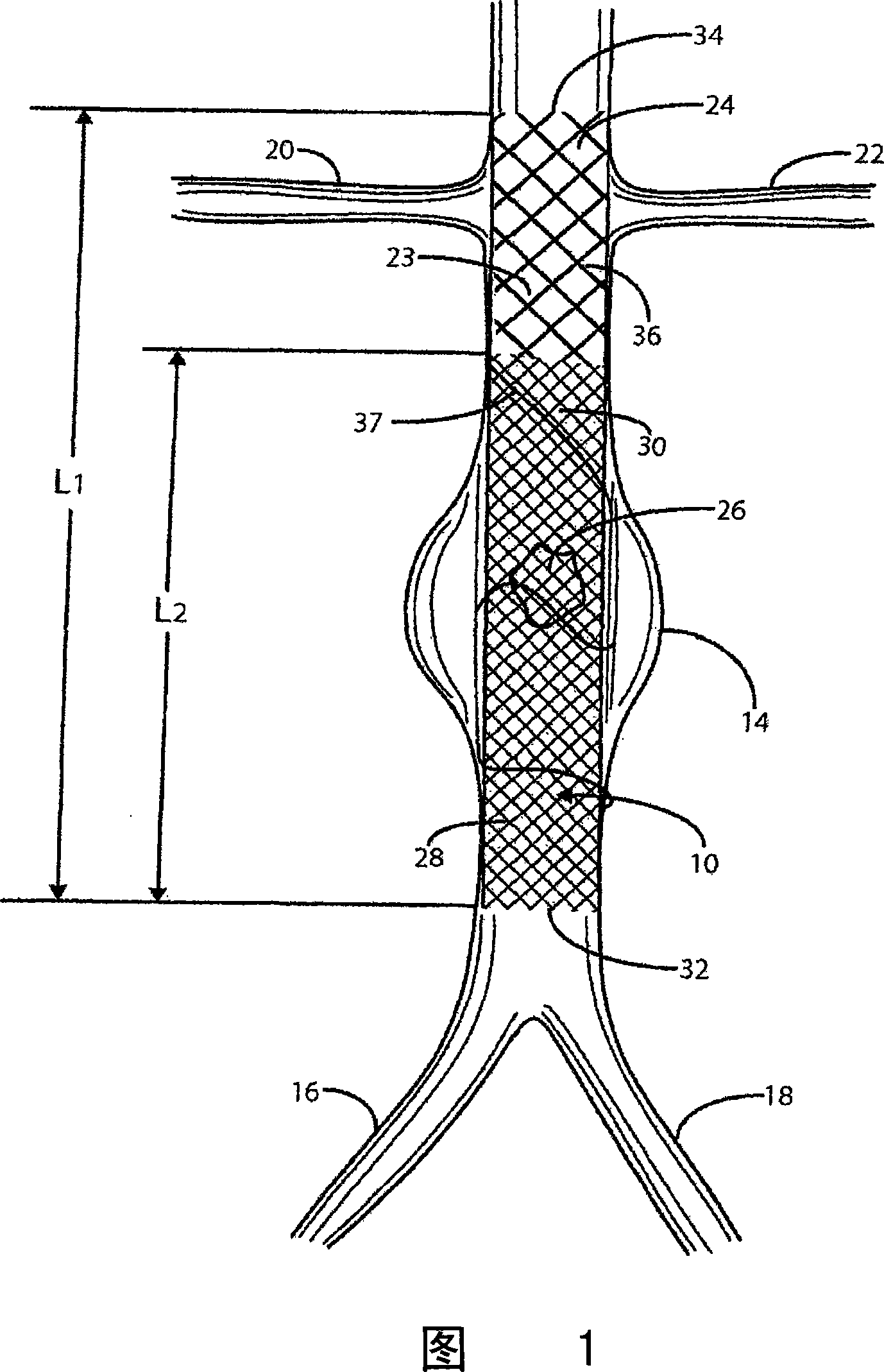
[0043] Referring to Fig. 1, numeral 10 indicates a stent / graft structure of a preferred embodiment of the present invention. Stent / graft 10 is shown deployed in a segment of abdominal aorta 12 containing an aneurysm 14 . At its lower end the abdominal aorta 12 branches into left and right iliac arteries 16 and 18 . Figure 1 also shows renal arteries 20 and 22 leading to the kidneys (not shown).
[0044] Stent / graft 10 comprises an innermost tubular member 23 of length L1, and at least one further tubular member 26 of length L2, both structures having similar predetermined diameters. The overall length of L1 of the stent / graft is 12-16 cm, preferably 14 cm; the length of L2 is 8-12 cm, preferably 10 cm.
[0045] The innermost tubular member comprises a first plurality of braided wire strands 24, preferably a shape memory alloy such as Nitinol. The braid comprises an innermost tubular member 23 having a predetermined weft density and pick and pitch to define a sufficiently la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com