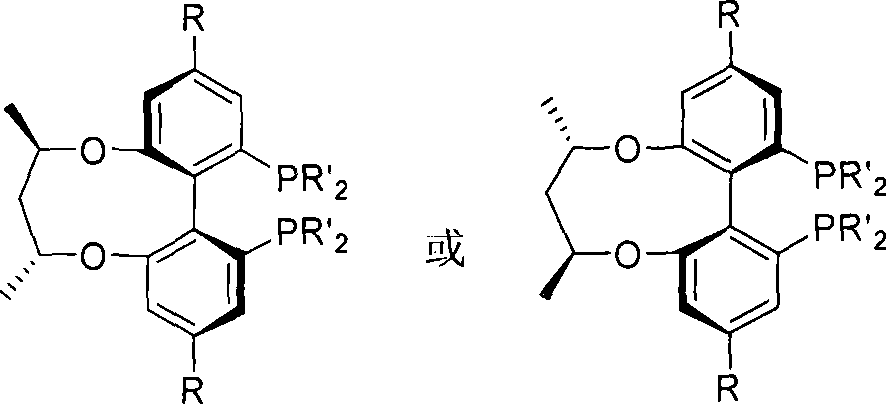
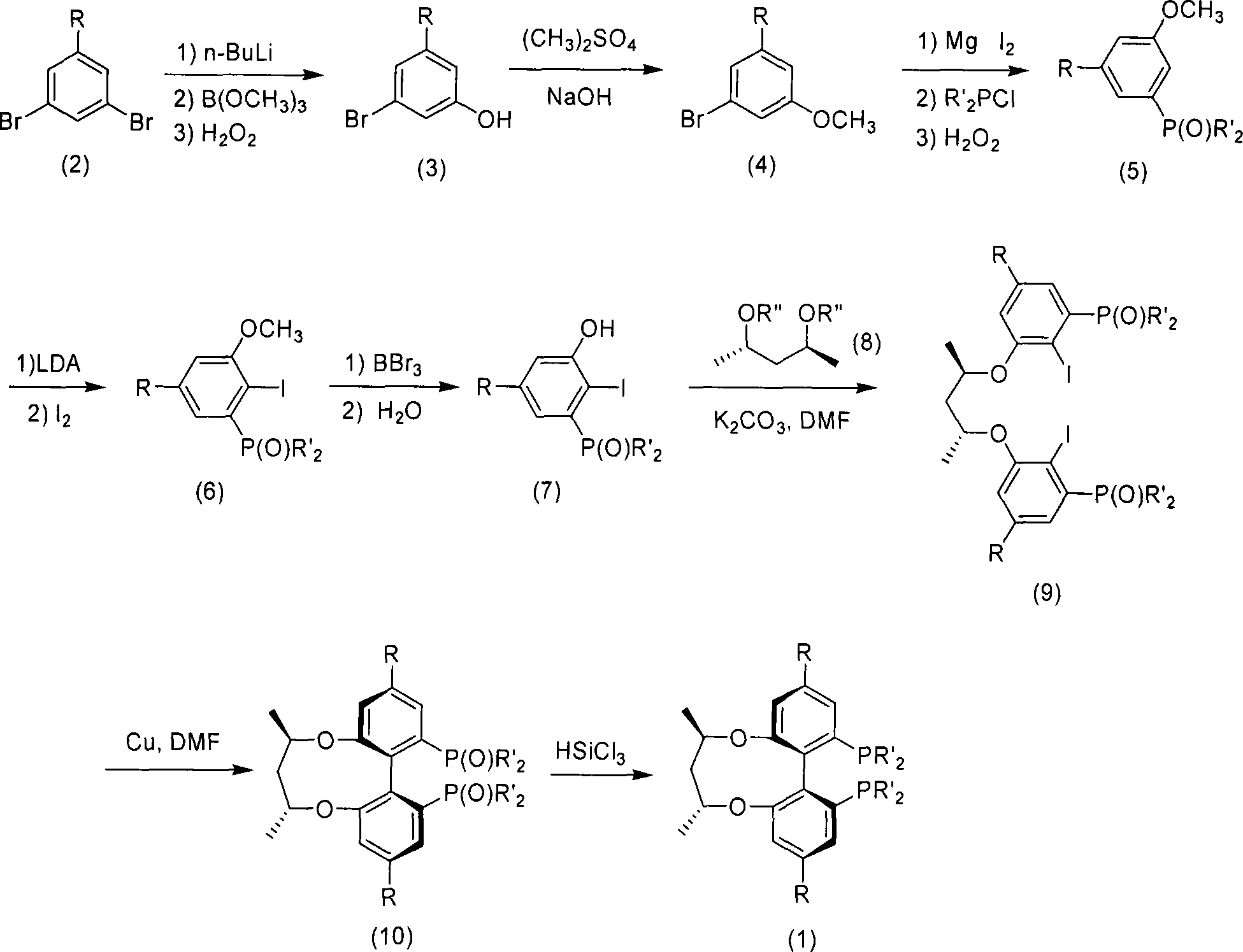
Central chirality induced axial chirality diphosphine ligand and method for synthesizing same
A technology of chiral induction and bisphosphine ligands, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as unsatisfactory resolution results, and achieve easy raw materials, The synthesis process is simple, the effect of simplifying the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 3-tert-butyl-5-bromophenol (3a)
[0033] Under nitrogen protection, 25.017 g (about 85.7 mmoL) (1-tert-butyl-3,5-dibromobenzene 2a) and 200 mL of THF were added to the Schlenk bottle. At -80°C, add 42.8mL (2.00M, about 85.7mmoL) of n-BuLi, and lithiate for 3h. Add B(OC 2 h 5 ) 3 14.5mL (about 85.7mmoL), after stirring for 4h, add H 2 o 2 9mL and 1mL of NaOH solution (10moL / L). After stirring for 1h, add NaOH 2 SO 3 6.5g stirred. with saturated NH 4 Cl neutralizes, CH 2 Cl 2 Extract and spin dry to obtain crude product. Distillation under reduced pressure gave 16.662 g of compound (3a), with a yield of 84.9%.
[0034] 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.27(s, 9H), 6.80(d, J=11.4Hz, 2H), 7.08(s, 1H)\; 13 C NMR (CDCl 3 , TMS, 75MHz) δ31.07, 34.87, 111.71, 115.79, 121.26, 122.45, 154.99, 156.12; HRMS Calcd.for C 10 h 13 BrO: 229.1136, found: 230.1436;
Embodiment 2
[0036] Synthesis of 1-tert-butyl-3-bromo-5methoxybenzene (4a)
[0037] Dissolve 21.106g (0.09moL) of (3a) and 3.603g of NaOH (0.09moL) in 80mL of water, in an ice-water bath, stirring constantly, add 8.6mL (90mmoL) of (CH 3 ) 2 SO 4 , rise to room temperature after 1h. use CH 2 Cl 2 Extract and spin dry. Distillation under reduced pressure yielded 19.813 g of compound (4a), with a yield of 88.4%.
[0038] 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.28(s, 9H), 3.78(s, 3H), 6.85(s, 2H), 7.10(s, 1H); 13 C NMR (CDCl 3 , TMS, 75MHz) δ31.11, 34.93, 55.40, 111.26, 113.40, 121.28, 122.53, 154.58, 160.08; HRMS Calcd.for C 11 h 15 BrO: 243.1402, found: 266.1047;
Embodiment 3
[0040] (5a) Synthesis of (3-diphenylphosphineoxy-5-tert-butylanisole)
[0041] In the there-necked flask equipped with dropping funnel and reflux condenser, magnetic stirring, add 1.656g (57.5mmoL) Mg, a little I 2 and 70 mL of THF. Under slightly boiling conditions, the mixed liquid of 50mL of THF and 13.984g (57.5mmoL) of (4a) was added dropwise, and it took 1h to complete the dropwise. Keep boiling for 3h, the solution is yellow or earthy gray. In an ice-water bath, add 10.4 mL (57.5 mmoL) of PPh 2 Cl, after 3h, ice-water bath, add 13mL (125mmoL) of H 2 o 2 . After 4h, add Na 2 SO 3 19g stirred. use CH 2 Cl 2 Extract and spin dry to obtain crude product. with Et 2 Washed with O to obtain a white powder and 15.326 g of compound (5a). Yield 73.2%.
[0042] 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.24(s, 9H), 3.77(s, 1H), 6.99(d, J=13.8Hz, 10H), 7.10(s, 1H), 7.26(d, J=13.2Hz, 1H ); 13 C NMR (CDCl 3 , TMS, 75MHz) δ31.35, 35.17, 55.56, 113.41----159.67; HRMS Calcd.for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com