Method for identifying human blood albumin products
A technology for human albumin and products, applied in the field of human albumin, can solve the problems of unsuitability for on-site detection, high technical requirements, long inspection period, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Operation method
[0031] 0.2mol / L citric acid buffer (pH4.2): Mix 0.2mol / L citric acid and 0.2mol / L sodium citrate at a volume ratio of 12.3:7.7, add 100mg of sodium azide per liter of buffer , adjust the pH to 4.2 with 0.2mol / L citric acid and 0.2mol / L sodium citrate.
[0032] BCG reagent (0.15mmol / L): Weigh 105mg of bromocresol green, dissolve it in 2.5ml of 0.1mol / L NaOH (heating to aid dissolution), then add 0.2mol / LpH4.2 citrate buffer 1000ml , plus Tween-801 ~ 2ml.
[0033] Take 2ml of BCG reagent, add 1 drop of the test product, and shake well.
[0034] If the test product is 5% to 25% genuine human serum albumin, the color of the solution should immediately change from yellow to green, otherwise the test product is a counterfeit product.
Embodiment 2
[0035] Embodiment 2 Sensitivity experiment
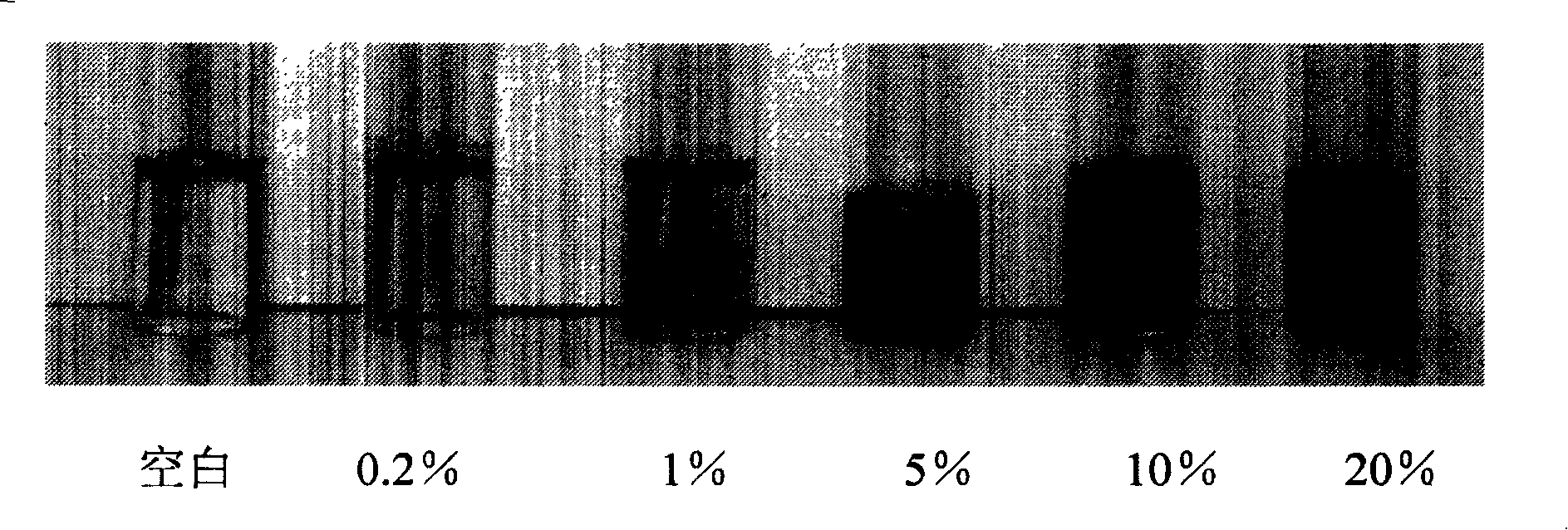
[0036] Such as figure 1 , six test tubes are equipped with 2ml BCG reagent, the first tube on the left is a blank control, and the remaining tubes are added dropwise from left to right with human serum albumin concentrations of 0.2%, 1%, 5%, 10%, and 20% respectively. %. It can be seen from the results that the higher the concentration of albumin, the darker the green color of the solution after reaction. Judging by naked eyes, the detection sensitivity of this identification method to human serum albumin is 1% albumin concentration.
[0037] Compared with the national standard identification method ("Chinese Pharmacopoeia" 2005 edition three appendices VIII C, VIII D), using this identification method can obtain 80 batches of human serum albumin authentic products and 16 batches of human serum albumin counterfeit products sampled in the market. The correct test conclusion is drawn, and the accuracy rate of the test conclusion is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com