Sulfated bile acid enzyme fluorescence capillary analytical method and enzyme fluorescence quantitative reagent kit
A sulfonation and bile acid technology, applied in the field of clinical biochemical analysis, can solve the problems of only one-time use, low sensitivity, reagent pollution, etc., and achieve the effects of short operation time, improved sensitivity, and reduced waste liquid.
Inactive Publication Date: 2010-12-01
SICHUAN UNIV
View PDF2 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The main problem with the existing SBA enzyme colorimetric assay is that there are too many types of enzymes and reagents used (BSS, β-HSD, 3-ketobile acid-Δ4-dehydrogenase, diaphorase, β-NAD, WST-1, ASOD, Tween20, sorbitol, 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), enzyme consumption is big, and cost is high, and sensitivity is lower, and used precious reagent can only be used once; And reaction product A The cuvettes are not completely soluble in water, and the quantitative cuvettes also have different degrees of reagent pollution after use. If they are reused, they must be washed repeatedly. This is still a problem for the application of automatic analysis devices.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Login to View More
Abstract
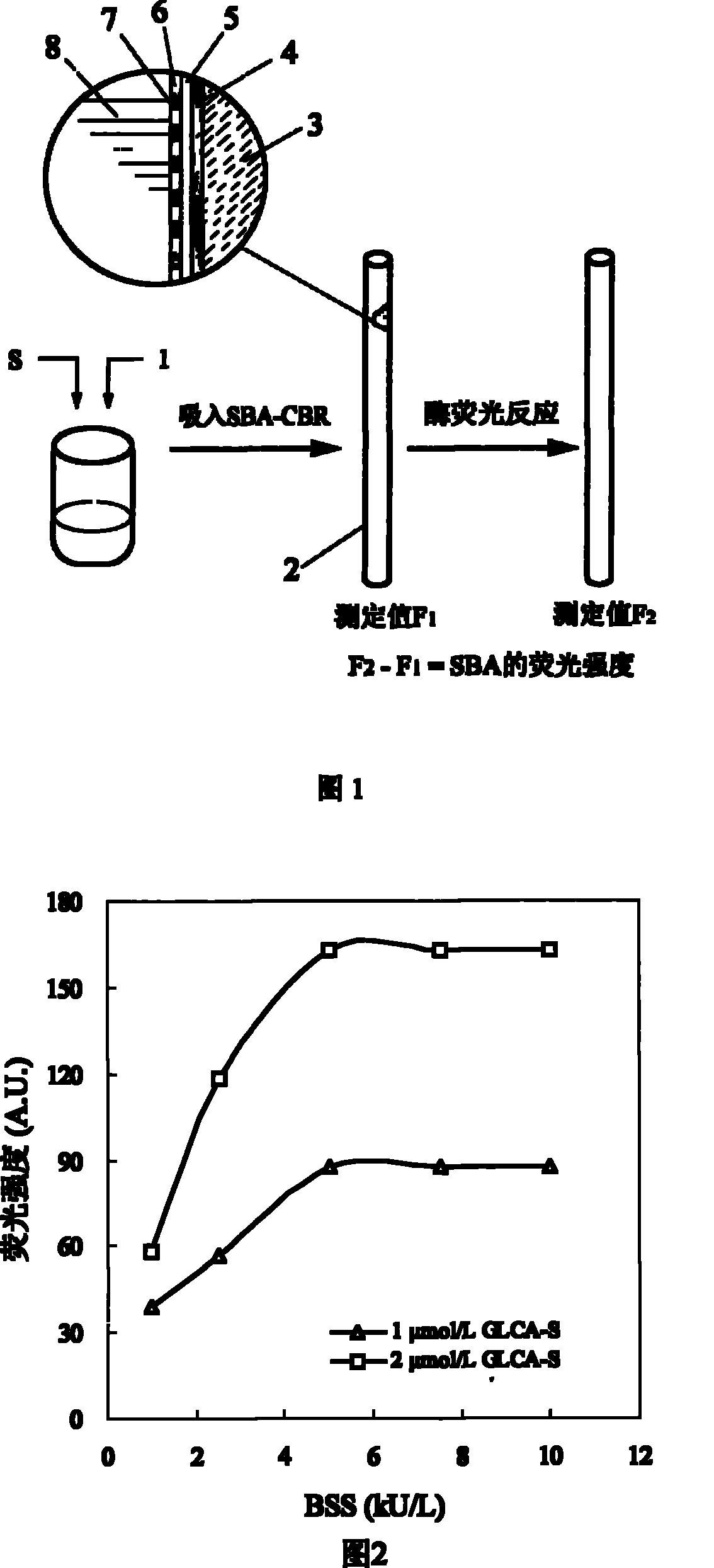
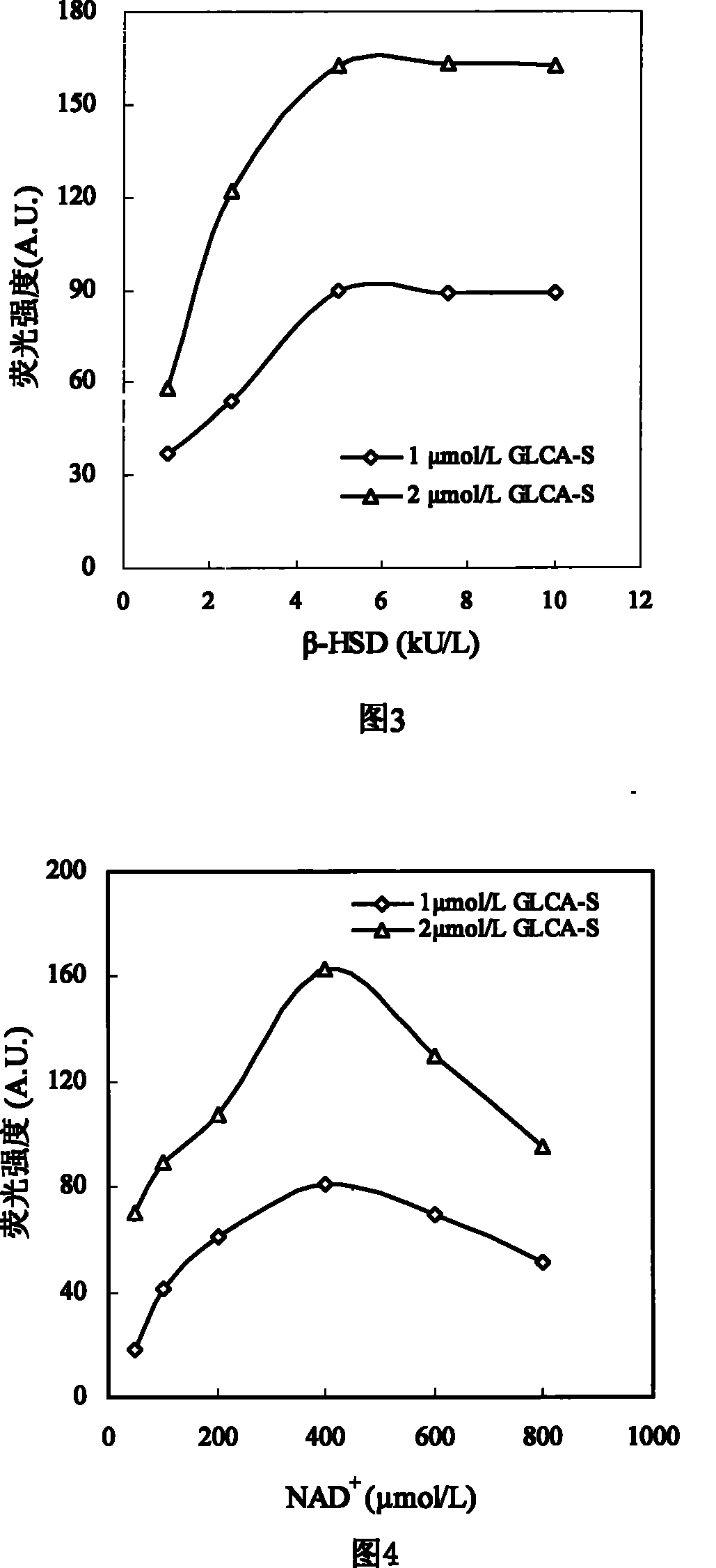
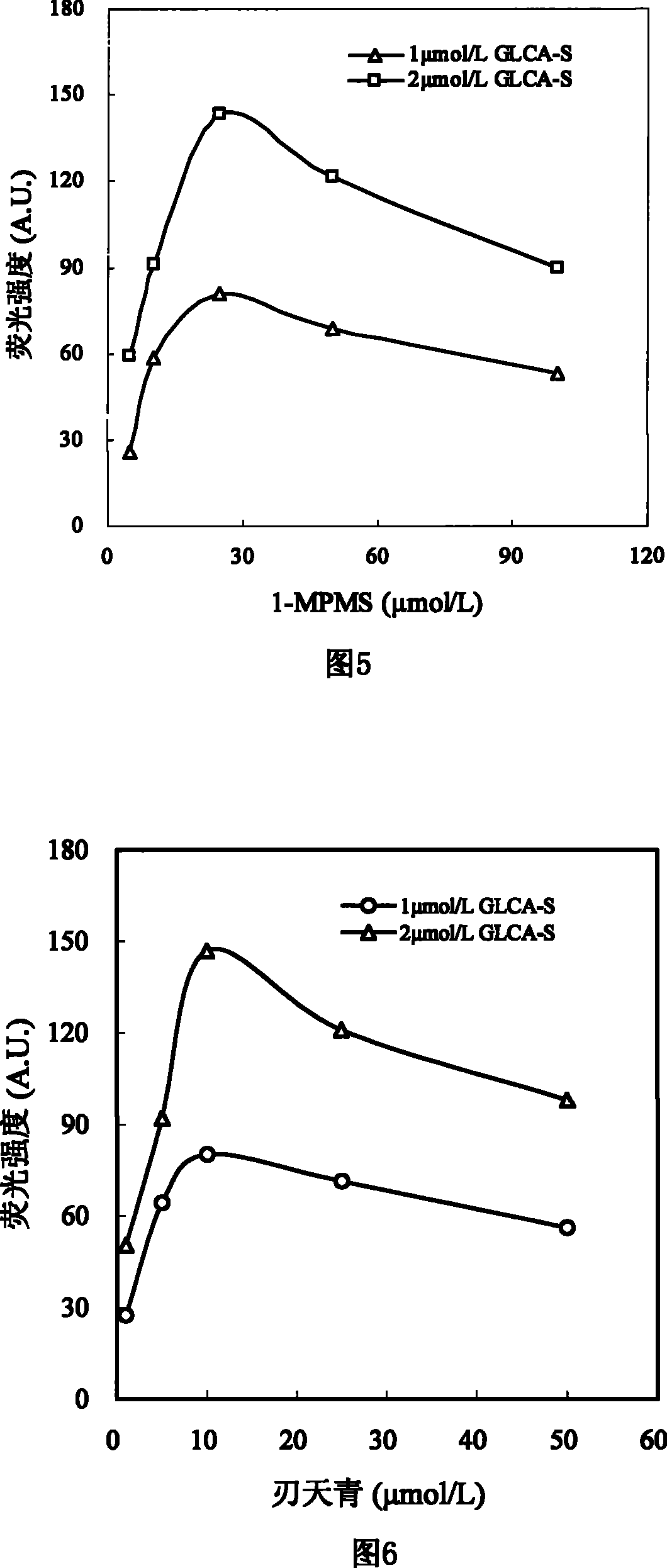
The invention discloses a sulfated bile acid (SBA) enzyme fluorescence capillary analysis and an SBA fluorescence quantitative test kit (SBA-FCA-Kit), which belongs to the field of a clinic biochemistry inspection field and is suitable for the fast screening and diagnosing of liver and gall diseases, more particularly, is suitable for the early period discovery of jaundice in neonatal period and congenital biliary atresia. After being mixed with fluorescence reaction liquid, a biology sample is sucked by an SBA capillary biology reactor (SBA-CBR) which contains sulfated bile acid sulfoacid esterase and Beta-hydroxysteroid dehydrogenase to determine the blank fluorescence intensity of the SBA-CBR under a given excitation wavelength and an emission wavelength; then the biology sample is ledto continuously react for a certain time and the fluorescence intensity is determined; the amount of the SBA in the sample is determined by using fluorescence intensity difference value. The SBA-FCA-Kit is composed of an SBA-CBR and fluorescence reaction liquid containing Beta-NAD<+>, an electric transmission body and diazoresorcinal, etc.
Description
Sulfonated bile acid enzyme fluorescence capillary analysis method and enzyme fluorescence quantitative kit technical field The invention relates to a sulfonated bile acidase fluorescence capillary analysis method and a SBA enzyme fluorescence quantitative kit for realizing the method, belonging to the field of clinical biochemical analysis. The invention is suitable for the general survey of the hepatobiliary system of healthy people, the rapid screening and diagnosis of the hepatobiliary diseases of clinical patients, and is especially suitable for the early detection of neonatal jaundice, congenital biliary atresia and hepatitis. Background technique Bile acids are synthesized in the liver. Under normal circumstances, 99% of bile acids exist in the enterohepatic circulation, and very little enters the systemic circulation. However, when the liver and gallbladder are damaged by disease, bile acids flow back into the blood, resulting in a significant increase in bile acid...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): G01N21/64
Inventor 李永生高秀峰
Owner SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com