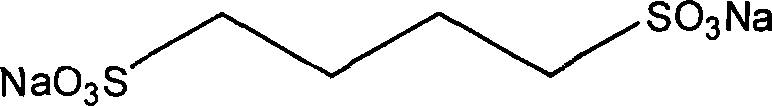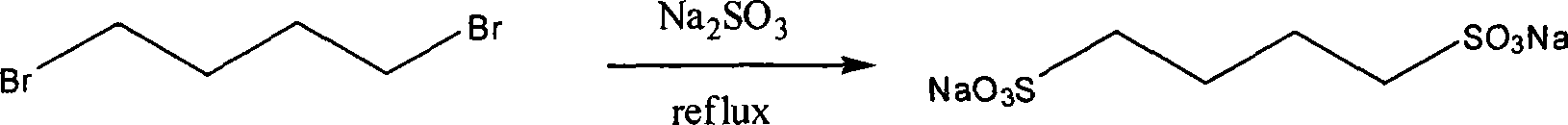Process for producing 1,4-sodium butanedisulfonic acid
A technology of sodium butanedisulfonate and sodium sulfite, applied in 1 field, can solve problems such as low yield, cumbersome post-processing, long reaction time and the like, and achieve the effects of high yield, easy operation and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: take 1,4-dibromobutane as raw material preparation 1,4-sodium butanedisulfonate
[0021] Add 7.56g (60mmol) of sodium sulfite, 3.6mL (30mmol) of 1,4-dibromobutane and 60ml of water into a 100ml single-necked bottle, heat and stir under reflux in an oil bath (temperature 110-120°C), and react for about 10 hours. About 50ml of water in the reaction system was distilled off under reduced pressure, cooled and crystallized at 4°C, and the obtained white crystals were recrystallized twice with water and dried under vacuum at 60°C to obtain sodium 1,4-butanedisulfonate. ESI-MS: 261.94; 1 H NMR (DMSO-d 6 ) δ ppm: 2.38 (t, J = 7.11, 4H), 1.59 (m, 4H). Yield: 95%.
Embodiment 2
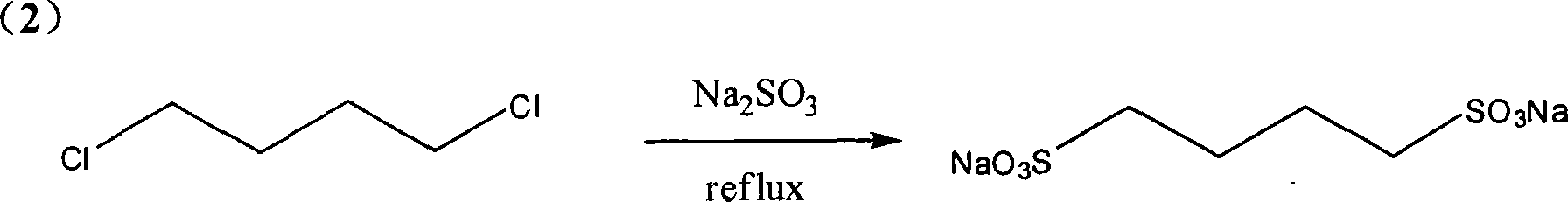
[0022] Embodiment 2: take 1,4-dichlorobutane as raw material preparation 1,4-sodium butanedisulfonate
[0023] Add 7.56g (60mmol) of sodium sulfite, 3.3mL (30mmol) of 1,4-dichlorobutane and 60ml of water into a 100ml single-necked bottle, heat and stir under reflux in an oil bath (temperature 110-120°C), and react for about 20 hours. About 50ml of water in the reaction system was distilled off under reduced pressure, cooled and crystallized at 4°C, and the obtained white crystals were recrystallized twice with water and dried under vacuum at 60°C to obtain sodium 1,4-butanedisulfonate. ESI-MS: 261.98; 1 H NMR (DMSO-d 6 ) δ ppm: 2.39 (t, J=7.11, 4H), 1.58 (m, 4H). Yield: 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com