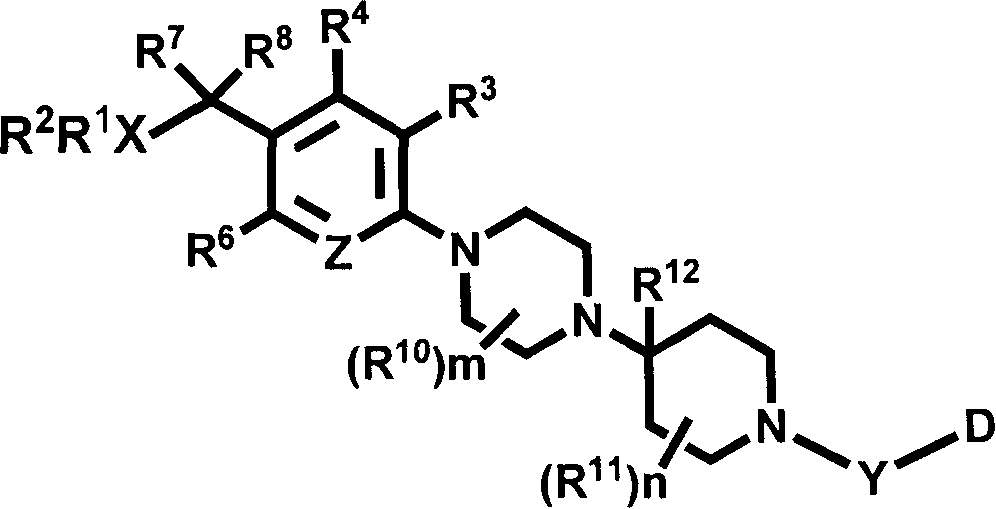
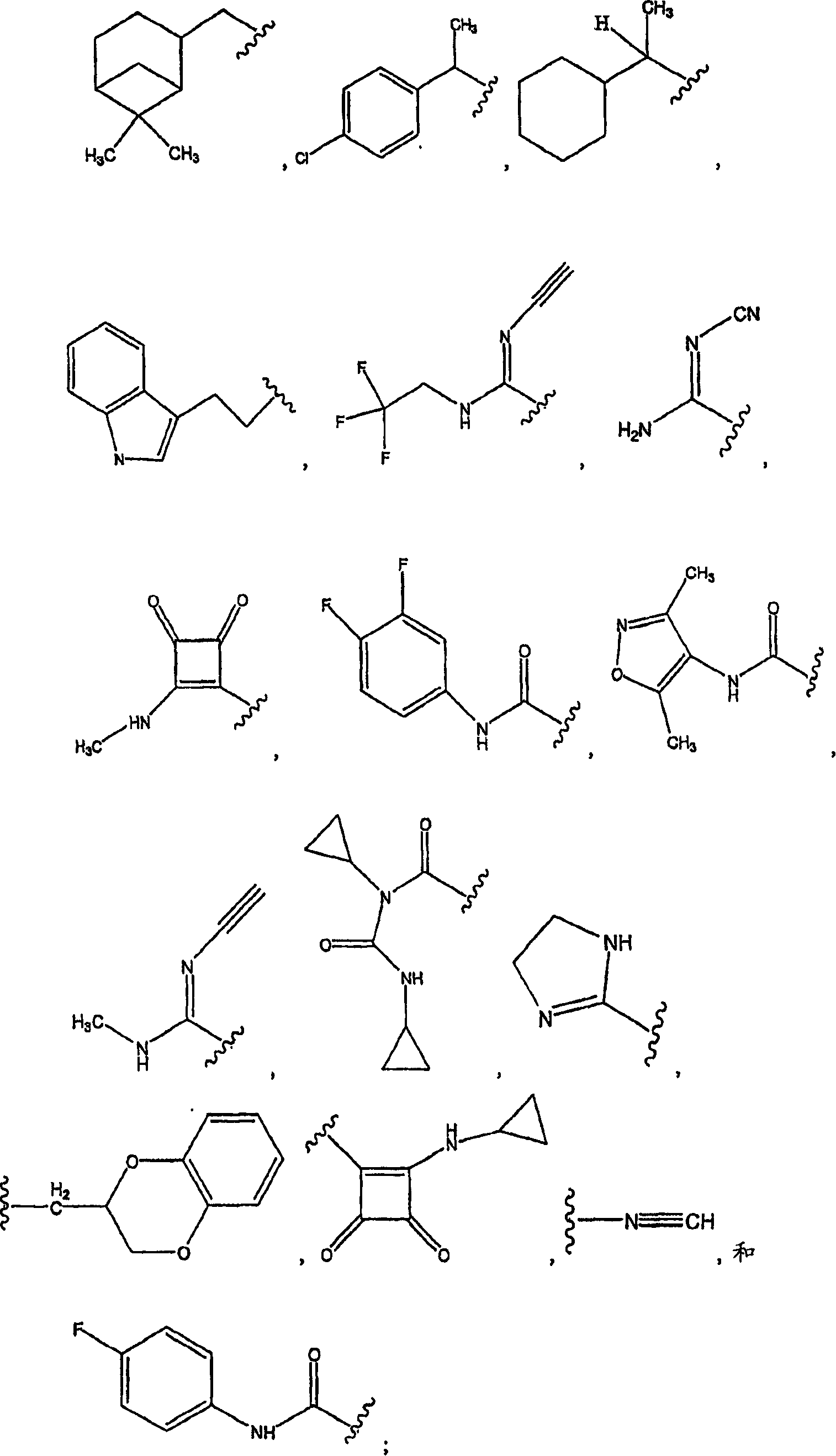
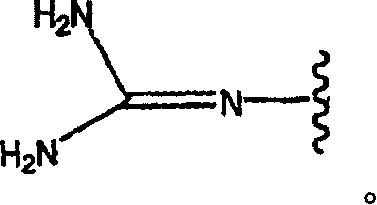
Pyridyl and phenyl substituted piperazine-piperidines with CXCR3 antagonist activity
A technology of R30 and R31, which is applied in the fields of antagonists and known immunity, and can solve problems such as undetected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0487]
[0488] To a round bottom flask was added 5,6-dichloronicotinic acid (30 g, 156 mmol), ethylamine HCl salt (14 g, 170 mmol), dimethylformamide (300 ml), dichloromethane (100 ml), triethylamine ( 26.1 ml, 187 mmol) and 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (32.9 g, 170 mmol). The reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo, the residue was dissolved in ethyl acetate (500 mL) and washed with 1N HCl (2x), saturated aqueous sodium bicarbonate (1x), brine (1x), dried over magnesium sulfate. The solution was filtered and concentrated in vacuo to give the desired 5,6-dichloro-N-ethyl-nicotinamide 2 (29 g, 85%). MS: M+H=219.
preparation Embodiment 2
[0490]
[0491] To a round bottom flask was added 5,6-dichloro-N-ethyl-nicotinamide (0.200 g, 1.3 mmol), Boc-2-(S)-ethyl-5-(R)-methyl-piperazine (0.200mg, 0.88mmol), potassium carbonate (1.2g, 8.8mmol) and dimethylformamide (5ml). The mixture was stirred overnight at 90°C. After filtration, the solvent was removed in vacuo and the residue was purified by flash chromatography to give the desired product 3 (0.172 g, 48%). MS: M+H=411.
preparation Embodiment 3
[0492] Preparation Example 3. Preparation of Compound No. 214 of Table 1
[0493]
[0494] Add intermediate to round bottom flask 3 (0.050 g, 0.161 mmol), dichloromethane (3 mL) and trifluoroacetic acid (1 mL). The mixture was stirred for 1 hour and the volatiles were removed in vacuo. Triethylamine (0.1 ml) and dichloromethane (1 mL) were added to the crude material to neutralize the TFA salt. Volatiles were removed in vacuo. To the residue was added 1-(4-chloro-benzyl)-piperidin-4-one (0.036 g, 0.32 mmol) and 3% acetic acid / dichloroethane. Add NaBH(OAc) 3 (0.102 g, 0.48 mmol), the mixture was stirred overnight at room temperature. After routine work-up, the crude product was purified by preparative HPLC to afford the desired compound 4 (0.030 g, 36%). MS: M+H=518.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com