Solid phase synthesis technique for melanotan-II
A technology of solid-phase synthesis and solid-phase peptide synthesis, which is applied in the field of synthesis of melanotan-II, can solve the problems of difficult synthesis, harsh protection conditions, etc., and achieve the effect of easy synthesis and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
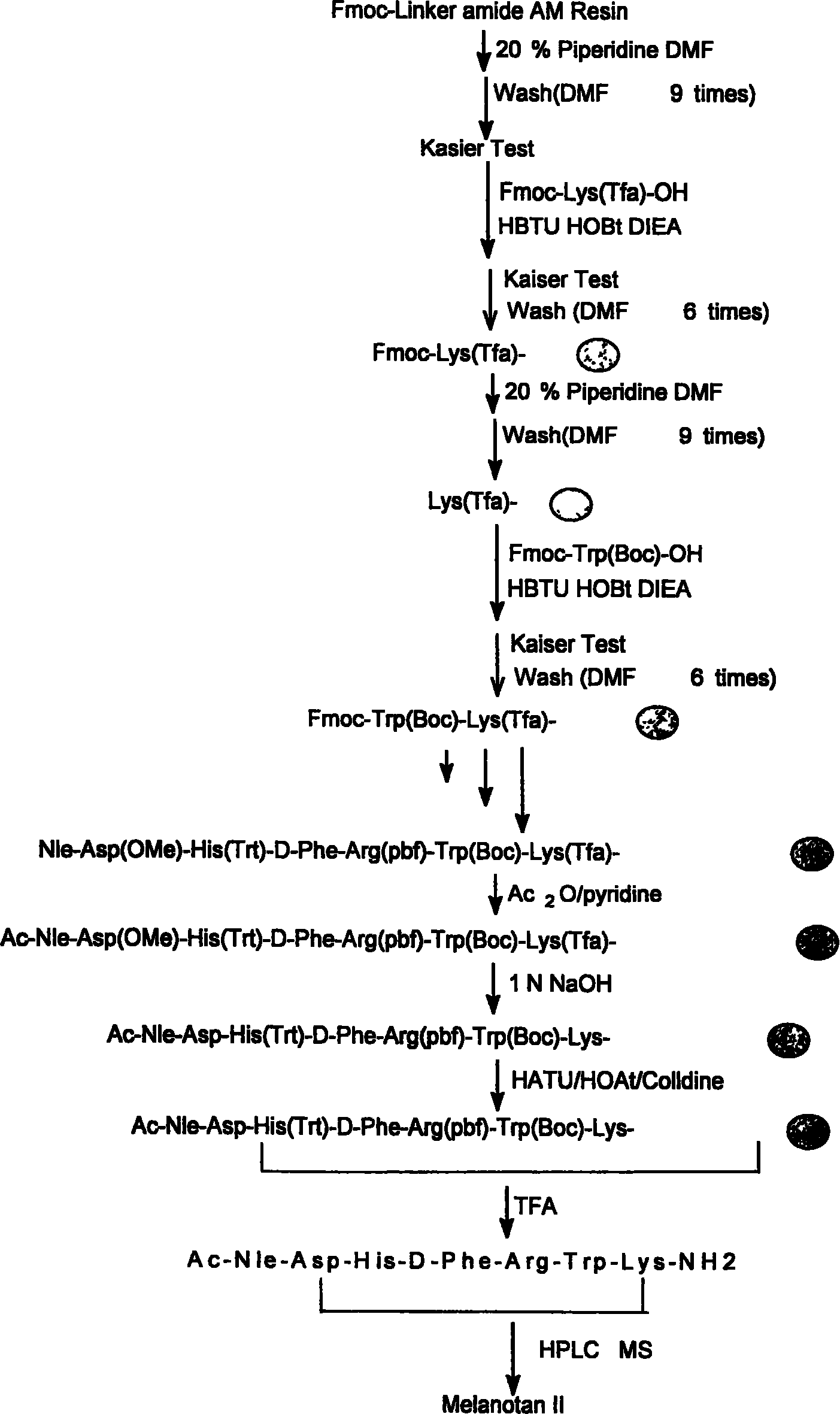
[0072] Add 1 gram of the resin obtained in step 1 into the peptide bottle, add 10 ml of 2N NaOH / dioxane (1:4, volume ratio) and react at room temperature for 4 hours, and the resin is mixed with DMF / water (1:1 , for volume ratio) three times, DMF three times, methanol three times, 2% glacial acetic acid / methanol (1:1, volume ratio) three times, methanol three times, dichloromethane three times. Kaiser test resin is bluish purple.
[0073] Take half of the resin with trifluoroacetic acid: water: sulfide anisole: dithioethanol (92.5: 2.5: 2.5: 2.5), shake at room temperature for 2 hours, filter, wash the resin twice with trifluoroacetic acid, concentrate the filtrate, add anhydrous ether , yellowish solid. After repeated washing with anhydrous ether, 296 mg of the crude product Ac-Nle-Asp-His-D-Phe-Arg-Trp-Lys-NH was obtained.
[0074] Molecular weight: 1042.22 ESI: 1042.74 (M + )521.82 (M + +2H)HPLC: 47.63%
example 2
[0076] Add 1 gram of the resin obtained in step 1 into a peptide bottle, add 10 ml of 1N NaOH / dioxane (1:1, volume ratio) and react at room temperature for 2 hours, and obtain 85 mg of crude product by the same method as Example 1. ESI: 1043.17 (M + ) HPLC: 52.46%
example 3
[0078] Add 1 gram of the resin obtained in step 1 into a peptide bottle, add 10 ml of 1N NaOH / dioxane (1:3, volume ratio) and react at room temperature for 2 hours, and obtain 102 mg of crude product by the same method as Example 1. ESI: 1043.09 (M + )522.54 (M + +2H)HPLC: 67.59%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com