Solid-liquid synthesizing method for leuprorelin
A technology for leuprolide and solid-liquid synthesis, which is applied in the field of solid-liquid synthesis of leuprolide, can solve the problems of inconvenient cutting of liquid ethylamine, synthesis time of unsafe liquid phase fragments, etc., and shorten the synthesis time, Avoid inconvenient cutting and simplify cumbersome effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
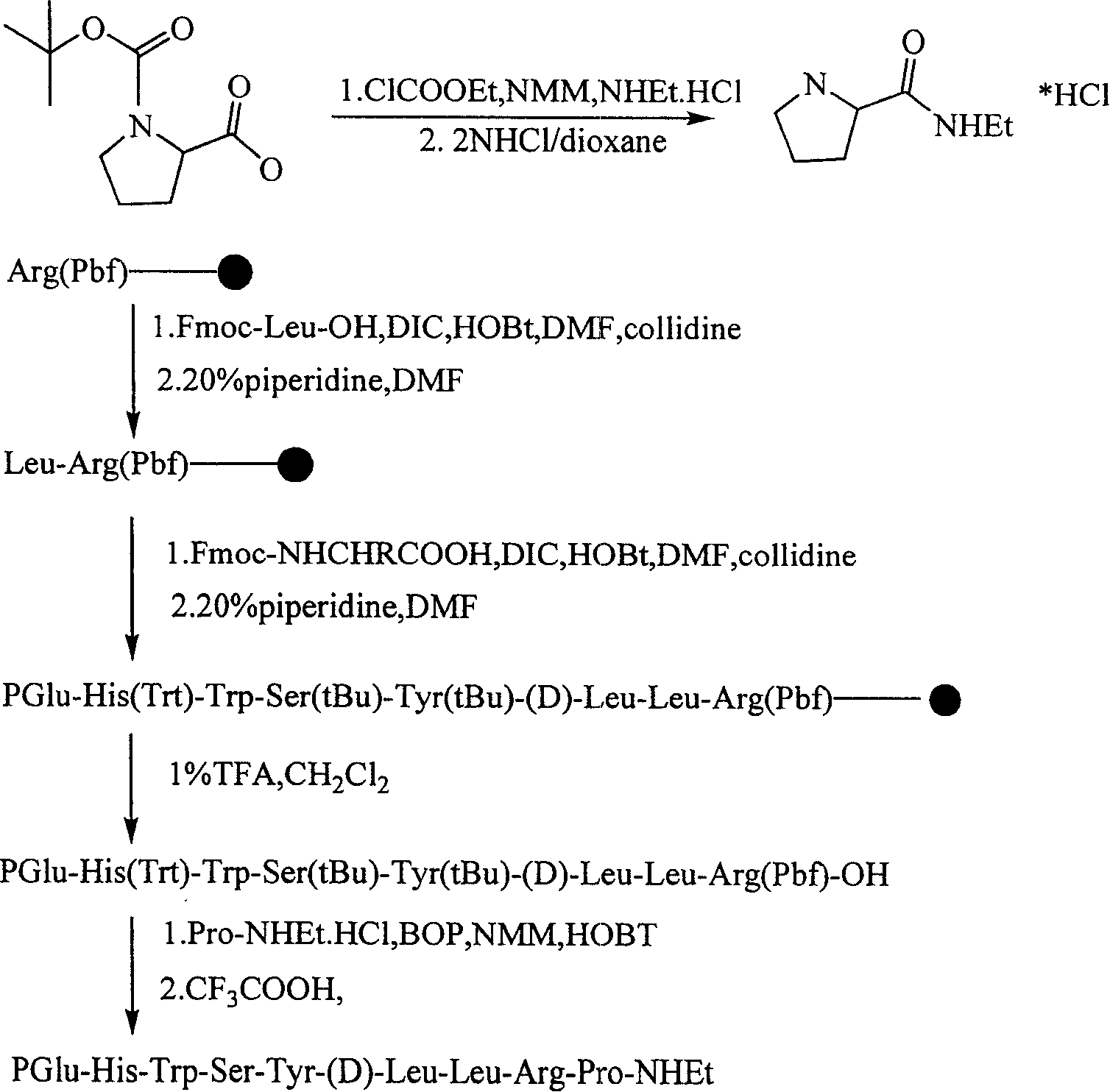
[0043] Example 1, with reference to the synthetic route, 5g of Arg(pbf).Trt(2-Cl)-ClResin (load: 0.4mmol / g) was placed in the peptide bottle and soaked in DMF solution, and Fomc-Leu-OH was added (molar ratio 1:3), DIC (molar ratio 1:1), HOBT (molar ratio 1:1), collidine (molar ratio 1:1), after shaking in a shaker at 25°C for 40min, wash 3 times with DMF, CH 2 Cl 2 Wash 3 times, then use 20% piperide-DMF solution to remove Fmoc, then use the same method to react sequentially until PGLu is connected, use 1% TFA-CH 2 Cl 2 The solution was cleaved to obtain 3gPGlu-His(Trt)-Trp-Ser(tBu)-Tyr(tBu)-(D)-Leu-Leu-Arg(Pbf)-OH. Dissolve 13g (60mmol) of BOC-Pro-OH in 100ml tetrahydrofuran, add 6.6ml (60mmol) of NMM, add 6ml (60mmol) of ethyl chloroformate at -10℃~-15℃, stir for 15~30min, then add ethylamine salt Acetate 4.8g (60mmol), stirred overnight at room temperature, after treatment to obtain the compound BOC-Pro-NHEt. The synthesized BOC-Pro-NHEt 6g was deprotected by 2mol / L dio...
Embodiment 2
[0044] Example 2, the fully protected polypeptide PGluR-8 (2mmol) and Pro-NHEt.HCl (2.2mmol) were dissolved in 100ml DMF, HOBt (2mmol), DIEA (4mmol) were added, stirred in an ice bath for 5-15min, and BOP ( 2mmol), after 25min, the ice bath was removed, and after stirring for 28 hours at 15°C, the fully protected peptide PGluP-9-NHEt was processed to obtain the fully protected peptide PGluP-9-NHEt, which was dissolved in the cutting solution (V / V%, trifluoroacetic acid: Cresol: water: thioanisole: thiol (82.5:5:5:5:2.5) was deprotected to obtain 1400 mg of crude leuprolide. All the other are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com