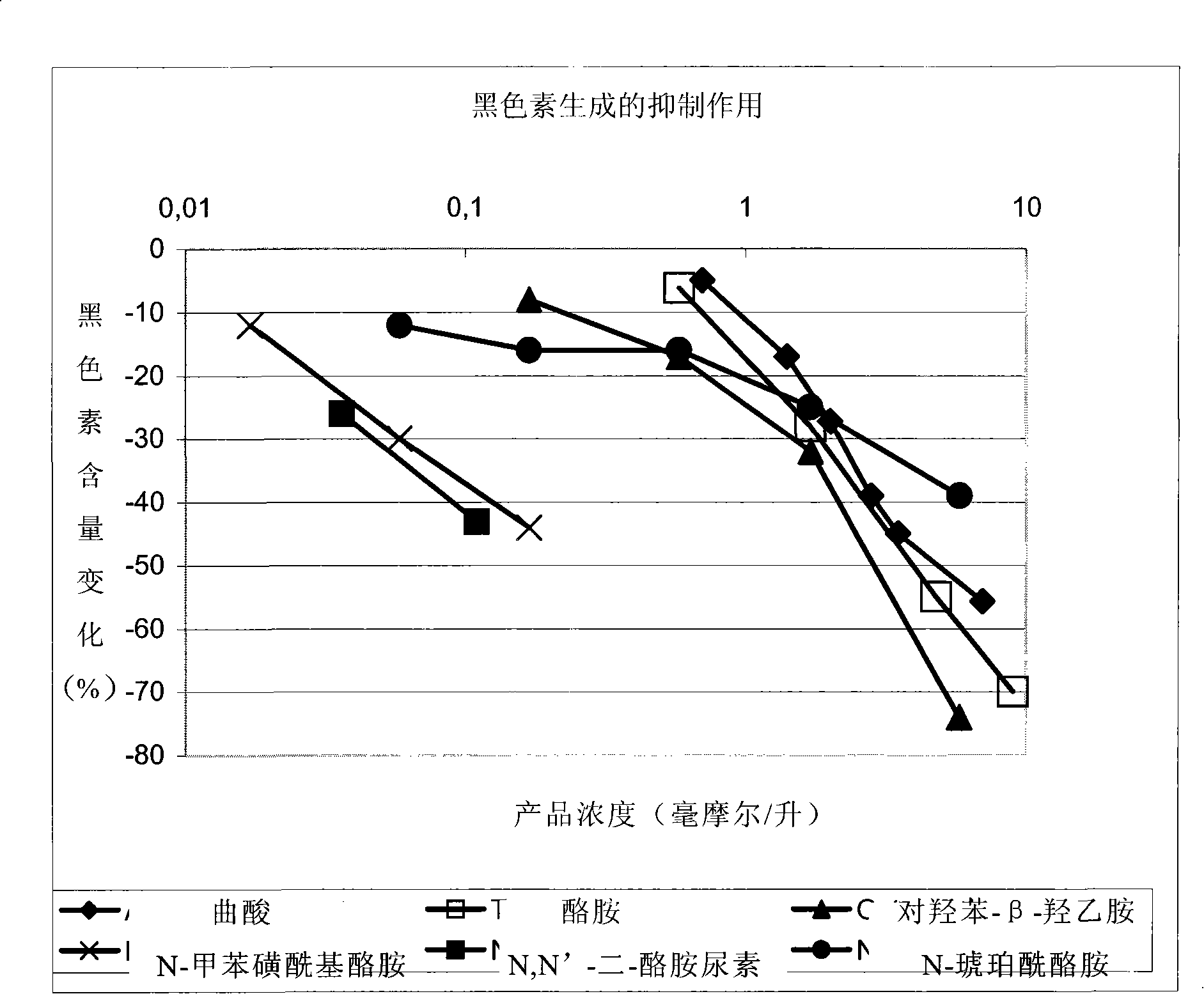
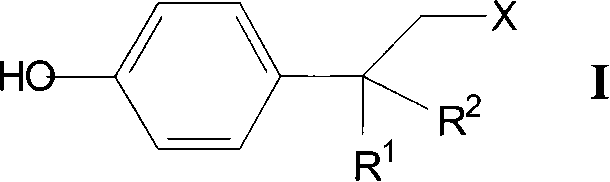
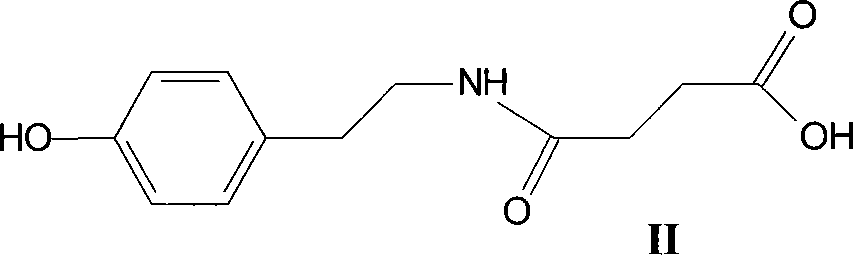
Mydrial derivates,preparation method of mydrial derivates and cosmetics or skin medicinal composition
A compound and derivative technology, applied in the field of new tyramide derivatives, can solve problems such as side effects and difficulty in application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0420] Example 1: Synthesis of N-succinyl-tyramide (compound II)
[0421] To a solution of tyramine hydrochloride (1.00 g; 5.76 mmol) in 20 ml THF was added 1 equivalent of potassium carbonate (K 2 CO 3 ) (0.80 g; 5.76 mmol) and 1.04 equivalents of succinic anhydride (0.60 g; 5.99 mmol). After stirring overnight at room temperature, water (10 ml) was added to hydrolyze the mixture, and 4 g of succinic resin IR120 (R-SO 3 H) Wash and stir for 15 minutes (pH=0-1). After filtering and washing with water, THF was evaporated coldly. 0.92 g (3.88 mmol; 67.3%) of N-succinyl-tyramide precipitated out as white crystals.
[0422] C 12 h 15 NO 4
[0423] MM=237.257gmol -1
[0424] Melting point: 135-136°C
[0425] CHN: Calculated: 60.75% C; 6.35% H; 5.90% N
[0426] Measured value: 61.33%C; 6.05%H; 5.86%N
[0427] Infrared spectrum: 3313; 3055; 2930; 1694; 1643; 1542; 1517; 1426; 1238;
[0428] 1208cm -1
Embodiment 2
[0429] Embodiment 2: the synthesis of N-toluenesulfonyl-tyramide (compound III)
[0430] Add 1 equivalent of potassium carbonate (K 2 CO 3 ) (0.16 g; 1.16 mmol) and 1.10 equivalents of tosyl chloride (0.12 g; 1.27 mmol). After stirring overnight at room temperature, water (4ml) was added to hydrolyze the mixture, and 4g of succinic resin IR 120 (R-SO3 H) Wash and stir for 1 hour. After filtering and washing with water, the solvent THF was then cold evaporated and the solid was filtered off. 0.19 g (0.65 mmol; 56.00%) of N-tosyl-tyramide precipitated out as white crystals.
[0431] C 15 h 17 NSO 3
[0432] MM=291.37gmol -1
[0433] Melting point: 169-172°C
[0434] CHN: Calculated: 61.83% C; 5.88% H; 4.81% N
[0435] Measured value: 61.94%C; 5.83%H; 4.78%N
[0436] Infrared spectrum: 3335; 3220; 1613; 1598; 1515; 1435; 1312; 1229; 1149; 1063; 913; 833; 812cm -1
Embodiment 3
[0437] Embodiment 3: the synthesis of N, N'-two-tyramine-urea (compound IV)
[0438] To a solution of tyramine hydrochloride (0.20 g; 1.15 mmol) in 4 ml THF was added 1 equivalent of potassium carbonate (K 2 CO 3 ) (0.16 g; 1.16 mmol) and 0.54 equivalents of carbonyl-diimidazole (0.10 g; 0.62 mmol). After two days of stirring at room temperature, the mixture was hydrolyzed by adding water (4ml) and THF (4ml), and 4g of succinic resin IR 120 (R-SO 3 H) Wash and stir for 15 minutes. After filtering and washing with 4 ml THF and then 4 ml water, the solvent was evaporated cold and the solid was filtered off. 0.09 g (0.27 mmol; 43.20%) of N,N'-di-tyramine-urea precipitated out as white crystals.
[0439] C 17 h 20 N 2 o 3
[0440] MM=300.3605gmol -1
[0441] Melting point: 95-98°C
[0442] Mass spectrometry: (m / z) = 301.2 [M+H] +
[0443] CHN: Calculated: 67.98% C; 6.71% H; 9.33% N
[0444] Measured value: 67.66%C; 6.73%H; 9.36%N
[0445] Infra Red: 3336; 3104; 293...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com