Remedy for disease associated with apoptotic degeneration in ocular cell tissue with the use of SIV-PEDF vector
一种组织细胞、药物的技术,应用在治疗青光眼领域,能够解决尚无青光眼治疗、治疗方法不合适等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
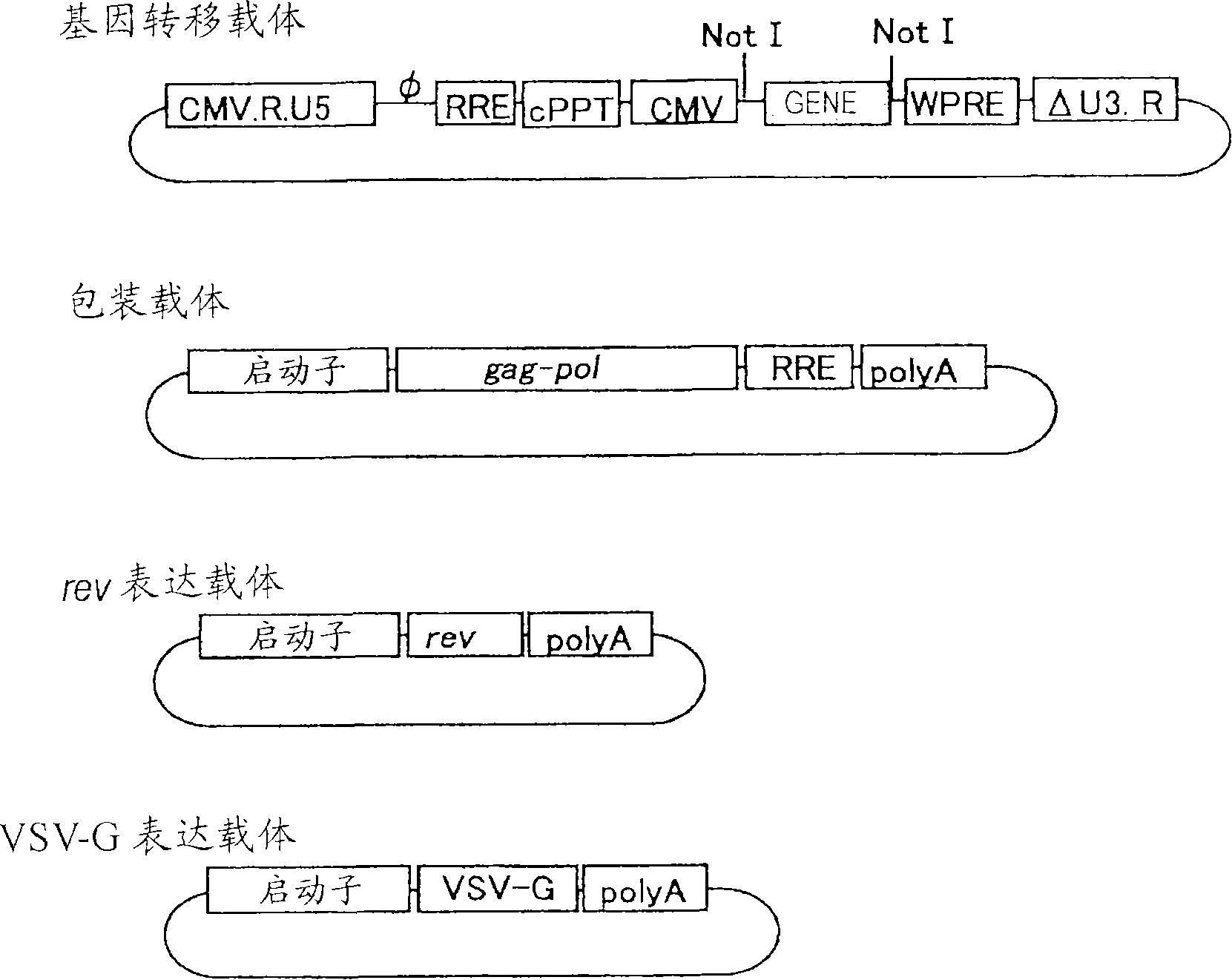
[0082] Example 1 Construction of VSV-G pseudotyped SIV vector
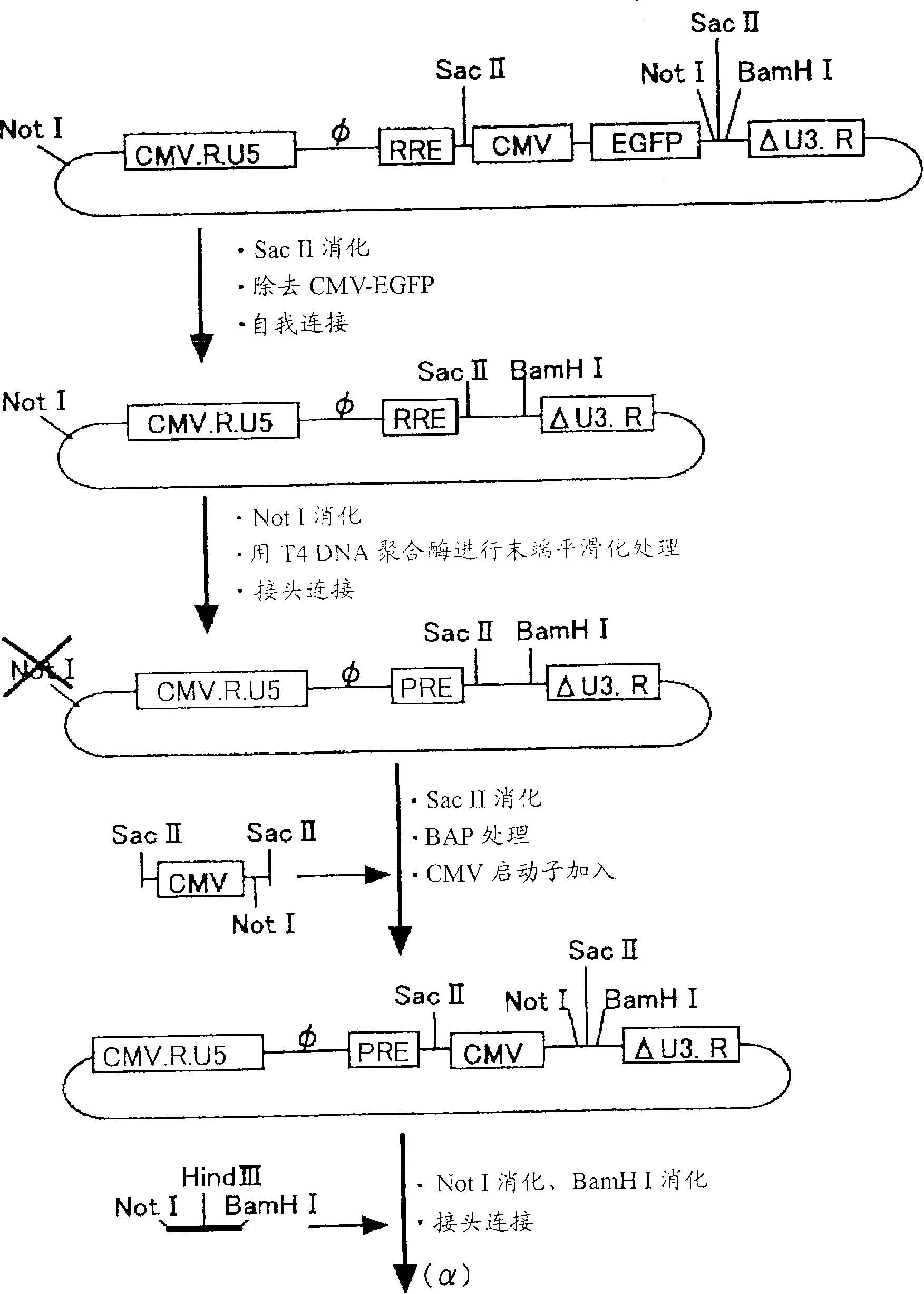
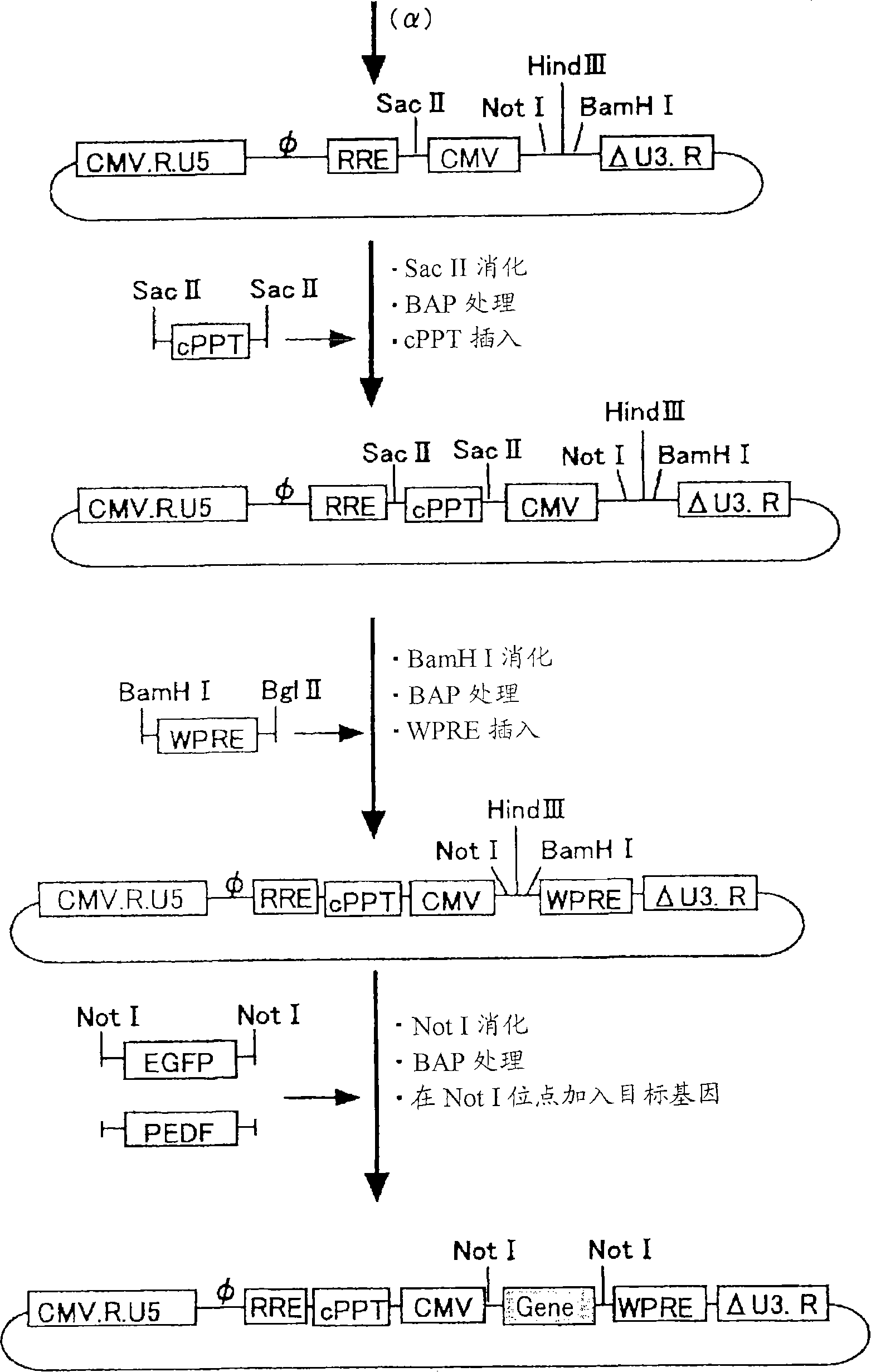
[0083] Vectors were constructed using the figure 1 The 4 plasmids (gene transfer vector, packaging vector, rev expression vector, VSV-G expression vector) shown in . Regarding the three types of gene transfer vectors, packaging vectors, and rev expression vectors, they were prepared by transforming the original vector plasmid (PCT / JP00 / 03955). As for the VSV-G expression vector, an unmodified original vector was used.
[0084] For plasmid preparation, various commercially available kits were used. Products from New England Biolabs were used as restriction enzymes, and QIAGEN kits (QIAquick PCR purification kit, QIAquick NucleotideRemoval kit, QIAquick Gel extraction kit, Plasmid Maxi kit) were used for plasmid DNA extraction, purification, and recovery. PCR uses TaKaRa's EX Taq enzyme, and the primers used are synthesized by SIGMA GENOSYS JAPAN. Dephosphorylation of DNA termini uses TaKaRa's alkaline phosphata...
Embodiment 2
[0102] Example 2 Functional evaluation of the SIV vector carrying cPPT, WPRE
[0103] In order to investigate the introduction effect of cPPT and WPRE, in addition to the vectors carrying both cPPT and WPRE, a vector carrying cPPT alone and a vector carrying WPRE alone were also produced, and compared with the original control. All gene transfer vectors used carried EGFP. The packaging carrier uses the original type (serial number: 27).
[0104] 2-1. Preparation of SIV vector
[0105] The cell line 293T cells from human fetal kidney cells were divided into about 1×10 per 15cm plastic culture dish 7 Inoculate (70-80% density on the next day) and culture in 20 ml of D-MEM medium (Gibco BRL) containing 10% fetal bovine serum for 24 hours. After 24 hours of cultivation, the medium was replaced with 10 ml of OPTI-MEM medium (Gibco BRL) and used as transfected cells for later use.
[0106] Dissolve 6 μg of gene transfer vector, 3 μg of packaging vector, and 1 μg of VSV-G express...
Embodiment 3
[0118] Example 3 Mass preparation and concentration of SIV vectors carrying therapeutic genes
[0119] Such as figure 1 The SIV vector was prepared as follows based on the four plasmids shown in the modified gene transfer vector, packaging vector, rev expression vector, and VSV-G expression vector. The vector carrying the PEDF therapeutic gene is produced in units of 20 15cm dishes.
[0120] According to each 15cm plastic Petri dish about 1×10 7 The 293T cells were inoculated (at a density of 70-80% on the next day), and cultured in 20 ml of D-MEM medium containing 10% fetal bovine serum for 24 hours. After 24 hours of cultivation, the medium was replaced with 10 ml of OPTI-MEM medium for transfection. Dissolve 10 μg of gene transfer vector, 5 μg of packaging vector, 2 μg of rev expression vector, and 2 μg of VSV-G expression vector in 1.5 ml of OPTI-MEM medium for each culture dish, and then add 40 ul of PLUS Reagent reagent (Invitrogen) for stirring , at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com