3,5-di-(4-nitrophenylamino diazo) benzoic acid and preparation method thereof
A technology of diaminobenzoic acid and nitroaniline, applied in 3 fields, can solve the problem of low sensitivity and achieve the effects of good selectivity, simple preparation method and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
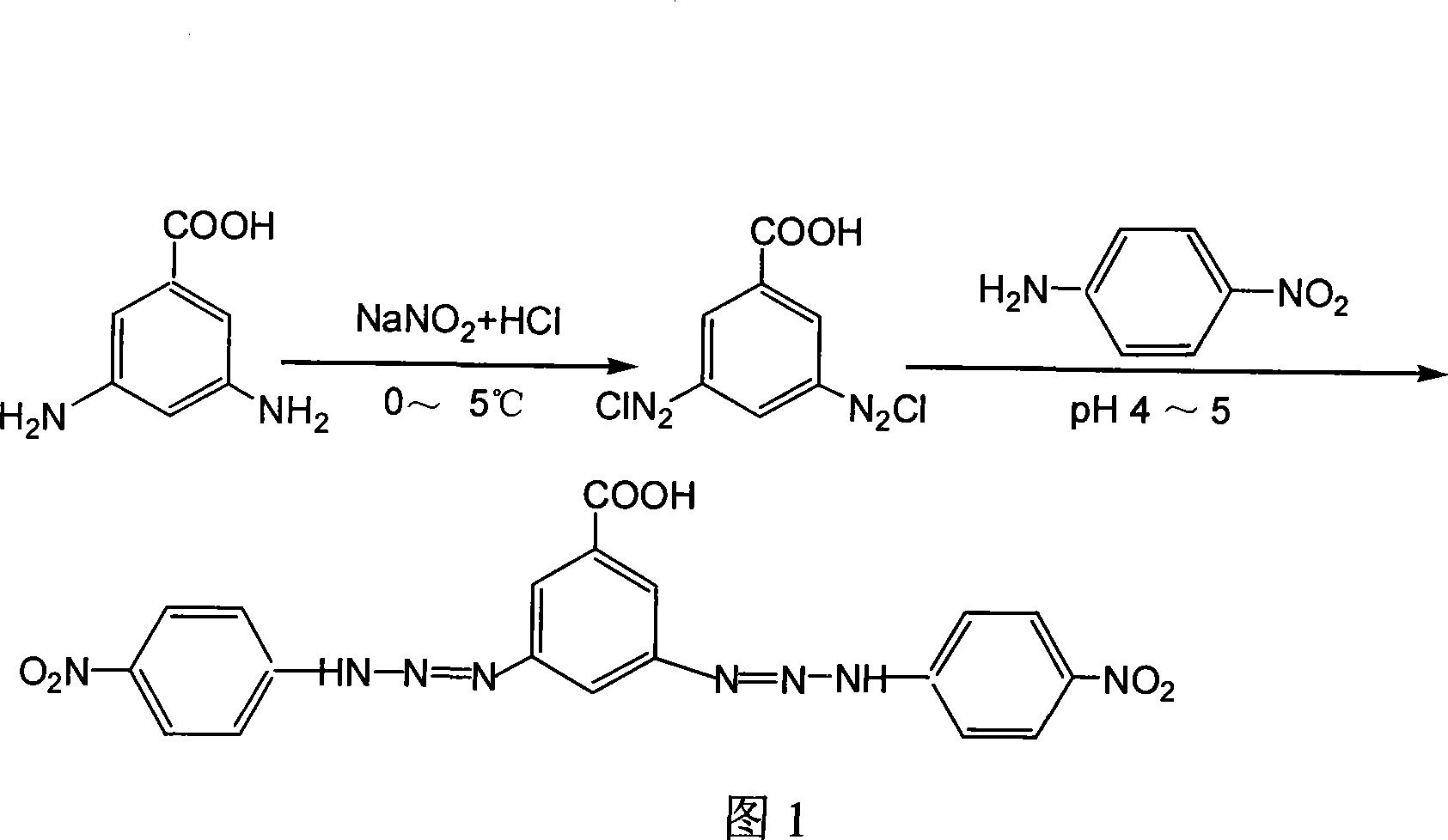
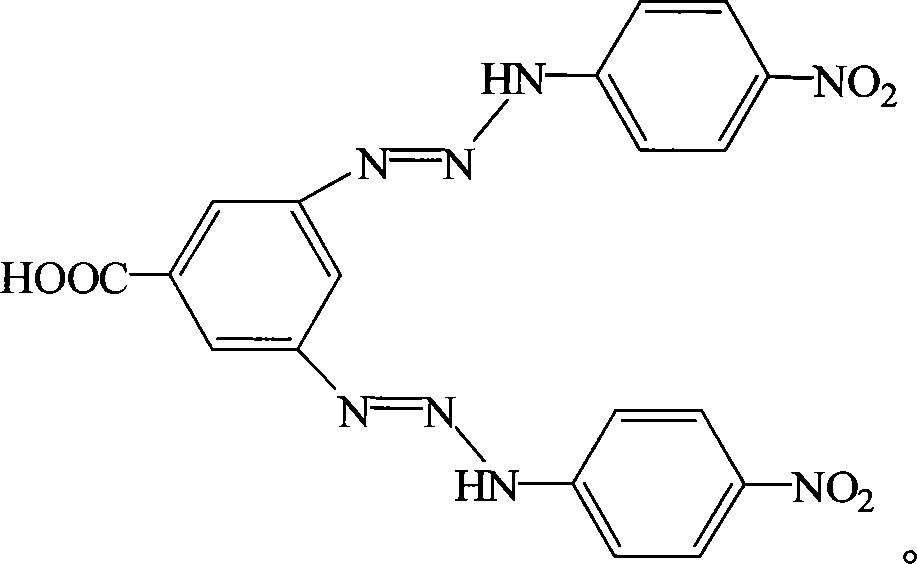
[0026] 3,5-di-(4-nitroanilinodiazo)benzoic acid (BNPADBP for short), its molecular structure formula:
[0027]
[0028] 3, the preparation method of 5-bis-(4-nitroanilinodiazo) benzoic acid comprises the following steps: (1) 7.6g (0.05mol) 1,3-diaminobenzoic acid is dissolved in 100ml 6mol / In the LHCl solution, put it in an ice-water bath at 0°C, weigh 7.0g (0.1mol) NaNO 2 Dissolve in 100ml of water, add to the above solution with stirring, continue to stir after the dropwise addition, and allow it to react at 5°C for about 2 hours to obtain a deep red suspension, namely the diazonium salt solution.
[0029] (2) 14 g (about 0.1 mol) of p-nitroaniline was dissolved in 300 ml of ethanol, and this solution was slowly added dropwise to the above-mentioned diazonium salt solution under stirring at 5°C. After the dropwise addition, use 20% NaOH solution to adjust the pH of the reaction solution to 4 to 5, adjust the pH to 6 to 7 after reacting at 2°C for 1.5 h, and obtain a lar...
Embodiment 2
[0031] Example 2, the steps are the same as in Example 1, the difference is that the temperature in step (1) is in an ice-water bath at 5°C respectively, and reacts at 0°C for 1h; the temperature in step (2) is successively 0~5°C, 9°C Reaction 1.5h.
Embodiment 3
[0032] Example 3, the steps are the same as in Example 1, the difference is that the temperature in step (1) is respectively 3° C. in an ice-water bath, and reacts at 2° C. for 1.5 h; the temperature in step (2) is respectively 4° C. and 5° C. respectively. 1.5h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com