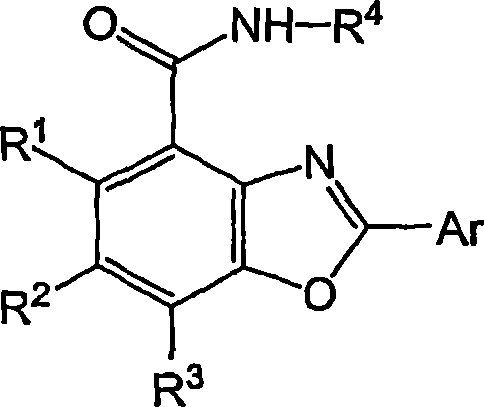
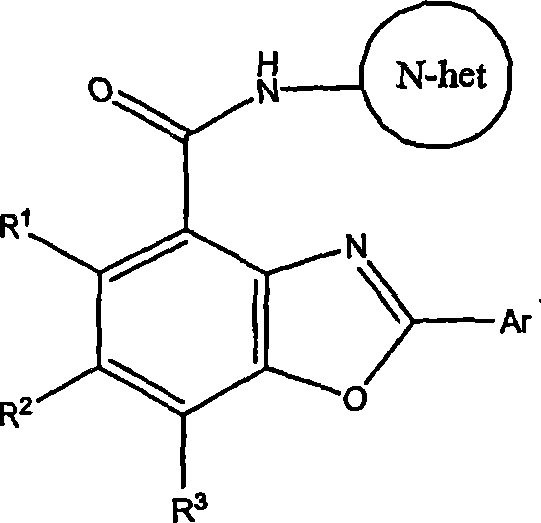
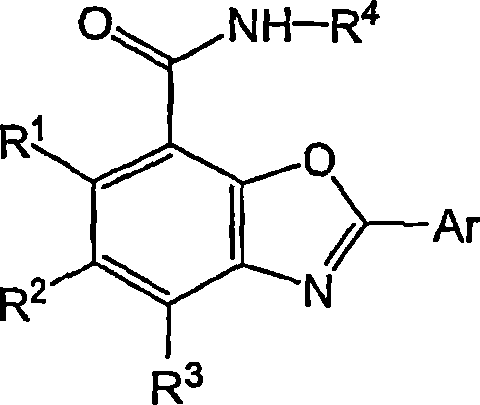
Benzoxazole carboxamides for treating cinv and ibs-d
A technology based on formamide and phenyl, which can be used in medical preparations containing active ingredients, allergic diseases, metabolic diseases, etc., and can solve problems such as reluctance to participate in social and family activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of N-[3-(2-methylimidazol-1-yl)propyl]-2-phenylbenzoxazole-4-formamide
[0077]
[0078] Step A: Benzoyl chloride (0.830 g, 5.90 mmol) was added to a suspension of 3-hydroxyanthranilic acid (0.300 g, 1.96 mmol) in toluene (10 mL) at room temperature, followed by pyridine (0.545 g, 6.92 mmol). The resulting mixture was stirred at room temperature for 30 minutes, then heated to 80 °C for 1 hour. The reaction was then cooled and poured into a mixture of ethyl acetate (50 mL) and 5% aqueous hydrochloric acid (50 mL). The layers were then separated and the organics were dried over magnesium sulfate and filtered to give an orange solution. The solution was concentrated to an orange solid, which was redissolved directly in xylene (20 mL), and the solution was treated with p-toluenesulfonic acid (0.800 g, 4.20 mmol). The reaction mixture was then heated to reflux for 6 hours. After this time the reaction was cooled and poured into water (50 mL), the ...
Embodiment 2
[0081] Example 2: Preparation of N-[2-(2-methylimidazol-1-yl)ethyl]-2-phenylbenzoxazole-4-carboxamide
[0082]
[0083] Step A: 2-(2-Methylimidazol-1-yl)ethylamine was prepared from 2-methylimidazole and 2-chloroethylamine hydrochloride using the procedure described in Example 1, Step B. This material was obtained in 47% yield as a pale yellow oil. 1 H NMR (300MHz, CD 3 OD) δ6.95(br s, 1H), 6.82(br s, 1H), 4.90(bs, 2H), 3.90(t, J=6.9Hz, 2H), 2.93(t, J=6.9Hz, 2H) , 2.35(s, 3H); MS(APCI) m / z 126[M+H] + .
[0084] Step B: Preparation of N-[2- (2-methylimidazol-1-yl)ethyl]-2-phenylbenzoxazole-4-carboxamide. The compound was obtained in 35% yield as a white solid: melting point 125-127°C; 1 H NMR (300MHz, CD 3 OD) δ8.11(dd, J=7.8, 1.0Hz, 2H), 7.96(d, J=7.8Hz, 1H), 7.73(d, J=8.2Hz, 1H), 7.63-7.52(m, 3H) , 7.42(t, J=8.0Hz, 1H), 7.13(s, 1H), 6.86(s, 1H), 4.22(t, J=5.8Hz, 2H), 3.87(t, J=6.0Hz, 2H) , 2.34(s, 3H); MS(APCI) m / z 347[M+H] + .
Embodiment 4
[0085] Example 4: Preparation of N-(1-methylpiperidin-4-yl)-2-phenylbenzoxazole-4-carboxamide
[0086]
[0087] Step A: A slurry of ammonium formate (10.2 g, 162 mmol) in water (4.4 mL) was added to a solution of 1-methyl-4-piperidone (1.84 g, 16.2 mmol) in methanol (40 mL), and The reaction was stirred until all solids had dissolved. Palladium on carbon (3.78 g, 10% Pd, 50% humidity) was then added and the reaction was stirred at room temperature for 18 hours. The mixture was then filtered through a celite pad, and the filtrate was concentrated under reduced pressure. The resulting viscous clear oil was dissolved in ethanol (33 mL), and the solution was treated with 37% hydrochloric acid (4.1 mL). After stirring at room temperature for 1 hour, the resulting solution was concentrated into a white solid, which was recrystallized from ethanol to obtain 0.62 g (yield 25%) of 4-amino-1-methylpiperidine dihydrochloride as a white solid: 1 H NMR (300MHz, DMSO-d 6 )δ10.95(br ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com