Method for synthesizing epichlorohydrin
A technology of epichlorohydrin and a synthesis method, applied in directions such as organic chemistry, can solve the problems of high requirements for equipment, inconvenient operation, and high purification costs, and achieve the effects of low production cost, convenient operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The synthetic method of epichlorohydrin is realized through the following steps:
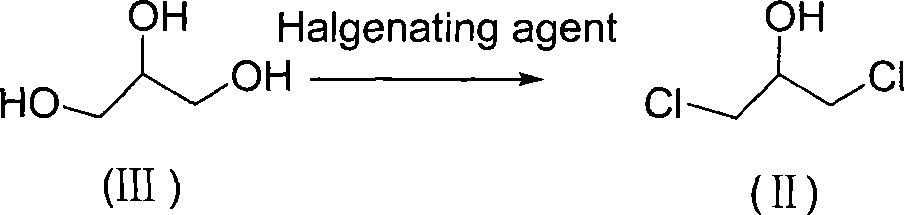
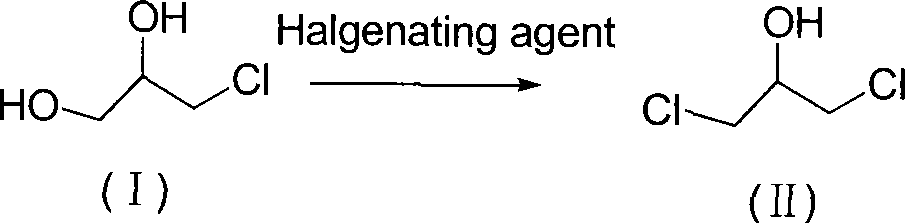
[0050] 1) Preparation of 1,3-dichloropropanol:
[0051] Add 110 g (1 mole) of R-3-chloro-1,2-propanediol and 13.6 g (0.1 mole) of zinc chloride into a 250 ml three-necked flask, raise the temperature to 90° C., and feed 3 moles of hydrogen chloride gas. ℃ for 5 hours, follow the reaction, when R-3-chloro-1,2-propanediol is completely converted to 1,3-dichloropropanol, cool, neutralize to neutral with calcium carbonate, filter, and use the mother liquor directly for Next reaction.
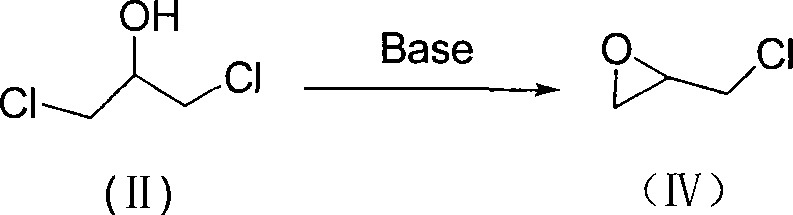
[0052] 2) Preparation of epichlorohydrin:
[0053] Add the 1,3-dichloropropanol prepared in the previous step to a 2500ml four-neck flask, add 1000ml of water, slowly add 130ml of 30% sodium hydroxide solution in portions, and pass water vapor under reduced pressure. Steam distillation, add lye, continue to react for 1 hour, steam distillation to no oil drops, and then separate the organic layer from the obt...
Embodiment 2
[0055] The synthetic method of epichlorohydrin is realized through the following steps:
[0056] 1) Preparation of 1,3-dichloropropanol:
[0057] Add 110 grams (1mol) of R-3-chloro-1,2-propanediol and 238 grams (2 moles) of thionyl chloride into a 250ml three-necked flask, react at room temperature for 2 hours, and neutralize with sodium carbonate until the pH is neutral. properties, filtered, and the mother liquor was directly used in the next step without purification.
[0058] 2) Preparation of epichlorohydrin:
[0059] Add 1,3-dichloropropanol in a 2500ml four-necked flask, add 1000ml of water, slowly add 130ml of 30% sodium hydroxide solution in portions, and feed water vapor under reduced pressure to carry out steam distillation. Finish the lye, continue to react for 2 hours, steam distill until there is no oil drop, and separate the organic layer from the obtained distillate, add an appropriate amount of sodium chloride to the water layer, separate the organic layer a...
Embodiment 3
[0061] The synthetic method of epichlorohydrin is realized through the following steps:
[0062] 1) Preparation of 1,3-dichloropropanol:
[0063] Add 110 grams (1mol) of R-3-chloro-1,2-propanediol and 59 grams (0.4 moles) of phosphorus oxychloride into a 250ml three-necked flask, react at room temperature for 2 hours, neutralize to neutral with sodium carbonate, After filtration, the mother liquor was directly used in the next reaction without purification.
[0064] 2) Preparation of epichlorohydrin:
[0065] In a 2500ml four-necked flask, add 1,3-dichloropropanol, add 1000ml of water, slowly add 130ml of 30% sodium hydroxide solution in portions, and under reduced pressure, let people steam to carry out steam distillation, After adding the lye, continue to react for 2 hours, steam distill until there is no oil drop, separate the organic layer until the obtained distillate is static, add an appropriate amount of sodium chloride to the water layer, separate the organic layer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com