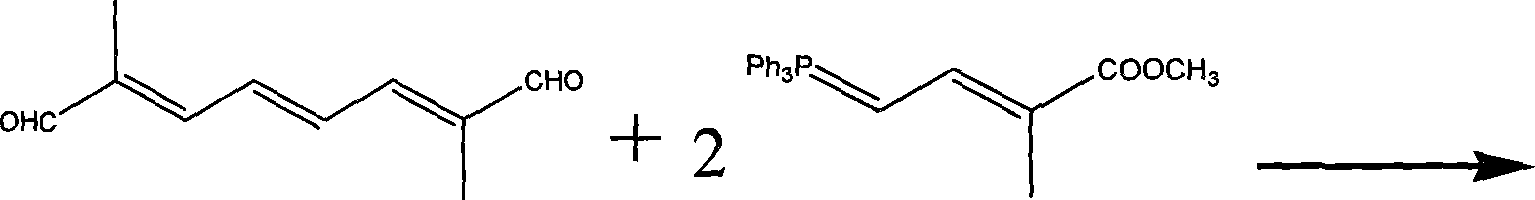Method for synthesizing saffron acid
A technology of saffron acid and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of difficult source of raw materials, unsatisfactory yield, difficult purification, etc., and achieve the effect of meeting market needs, good water solubility, and strong coloring power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
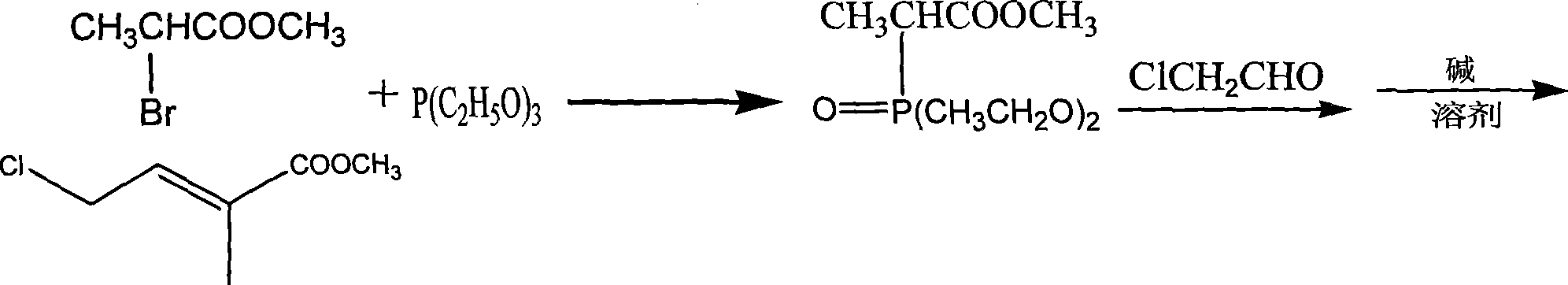
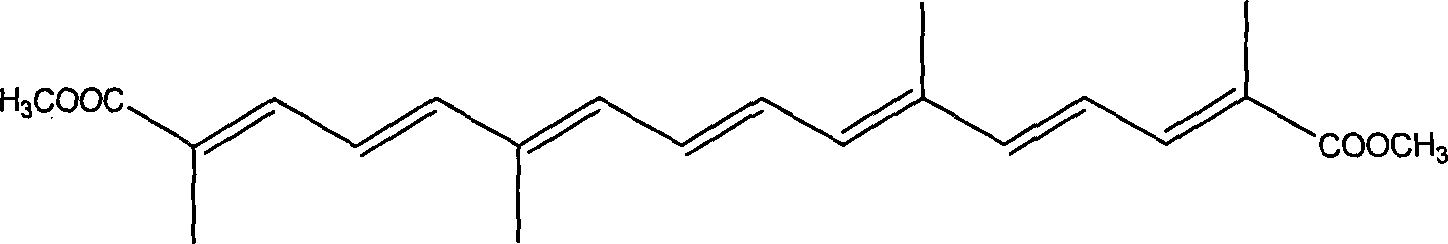
[0050]Add 150 mL of dimethylformamide and 32 grams of triphenylphosphine into a four-necked flask protected by nitrogen gas, heat to 80°C, add dropwise a mixed solution of 17 grams of methyl 2-bromopropionate and 50 mL of dimethylformamide, React for 12 hours, filter out the insoluble matter, dissolve the filtered insoluble matter in water, add potassium hydroxide to precipitate, wash and dry the precipitate, and recrystallize with ethyl acetate to obtain bromopropionate methyl bromide. Add 150 mL of isopropyl ether and the prepared bromopropionate bromopropionate bromopropionate bromopropionate bromopropionate 150 mL into a four-neck flask protected by nitrogen gas. When heated to 50 ° C, add 22 g of chloroacetaldehyde and 150 mL of isopropyl ether mixed solution and react for 2.5 h , recovered the solvent, and extracted the residue three times with 200 mL of anhydrous ether, combined the ether solution, concentrated and removed the ether to obtain 10.2 grams of methyl chlorot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com