Anticancer prior-medicine and method for preparing the same and use thereof
A technology of anticancer drugs and prodrugs, which is applied in the field of anticancer prodrugs and their preparation, and can solve the problems of unseen coupling of pectin and anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, high-ester pectin alkali method lowering ester
[0041] Weigh 10g of high-ester pectin, dissolve it in 1000mL of secondary water, adjust the pH to 10-13 with sodium hydroxide solution, keep at 10-60°C, stir and react for 30, 60, 90, 120, 150 and 180 min respectively, After the reaction, adjust the pH to 2.8-3.5 with concentrated hydrochloric acid, add 300ml of ethanol, centrifuge at 4500r / min for 15min, wash the precipitate with 60% ethanol solution to remove chloride ions, and dry at 100°C to obtain low-ester pectin.
Embodiment 2
[0042] Embodiment two, molecular weight segmentation
[0043] Pour the processed Sephadex G 75 into the chromatographic column while stirring, let it settle naturally for 20 minutes, and finally put a circular filter paper slightly smaller than the inner diameter of the chromatographic column, and carefully add the prepared pectin solution to the On the gel column, use a constant flow pump to adjust the secondary water flow rate to 4ml / tube / 6 minutes, measure the molecular weight distribution of pectin by GPC, and then combine pectin with a certain molecular weight range to obtain low-ester fruit with a molecular weight range of 1000-70000 glue.
Embodiment 3
[0044] Embodiment three, preparation one of pectin-doxorubicin conjugate
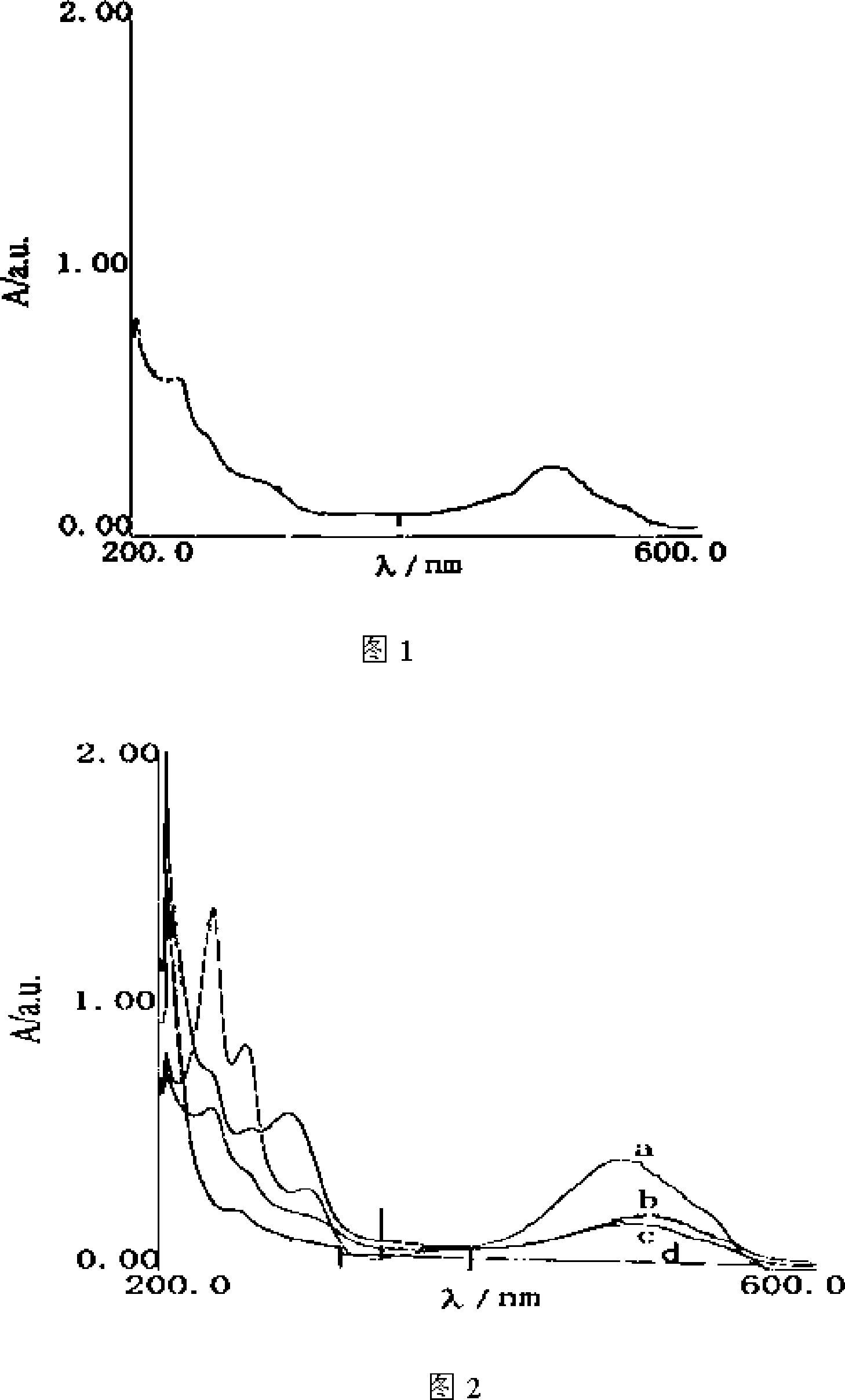
[0045] Weigh 100mg of low-ester pectin, dissolve it in a 100ml round bottom flask with 10ml of secondary water, and dissolve 50mg of doxorubicin hydrochloride in a mixed solution of 10ml of dimethylformamide / water (V / V=1:1) , temperature controlled at 30°C, and stirred for 30 minutes. Then 300mg of EEDQ was added, the temperature was controlled at 30°C, and the reaction was stirred for 24 hours. When the desired time is reached, the reaction mixture is transferred to a dialysis bag with a molecular weight cut off of 5000 and dialyzed against secondary water for 24 hours. Add 150 ml of absolute ethanol to the inner liquid of the dialysis bag to generate a precipitate, centrifuge, wash the precipitate with absolute ethanol, and dry to obtain a water-soluble conjugate. The UV diagram of the water-soluble conjugate of pectin and doxorubicin is shown in Figure 1, and the ultraviolet absorption of pectin, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com