Reagent kit for detecting epidermal growth factor acceptor third type mutant by real-time fluorescence quantitative PCR
An epidermal growth factor, real-time fluorescence quantitative technology, applied in fluorescence/phosphorescence, microbial determination/inspection, biochemical equipment and methods, etc., can solve problems such as disputes over the expression level of EGFRvIII, and achieve credible results and quantitative accuracy. High, improve work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Expression of EGFRvIII detected by fluorescence quantitative PCR
[0038] 1. Materials
[0039] Trirol reagent was purchased from Invitrogen, USA, and restriction endonuclease EcoR I was purchased from NewEngland, USA.
[0040] BioLabs, pGEM-T-Easy Cloning System, M-MLV Reverse Transcriptase, Oligo(dT) 15 It was purchased from Promega Company in the United States, and Tag DNA polymerase was a product of Bioflux Company in South Korea. The Model 377 sequencer was a product of Applied Biosystems in the United States, and the real-time fluorescent quantitative PCR detector and detection system version 2.0 were purchased from Hangzhou Bioer Technology Co., Ltd.
[0041] 2. Primer design and synthesis
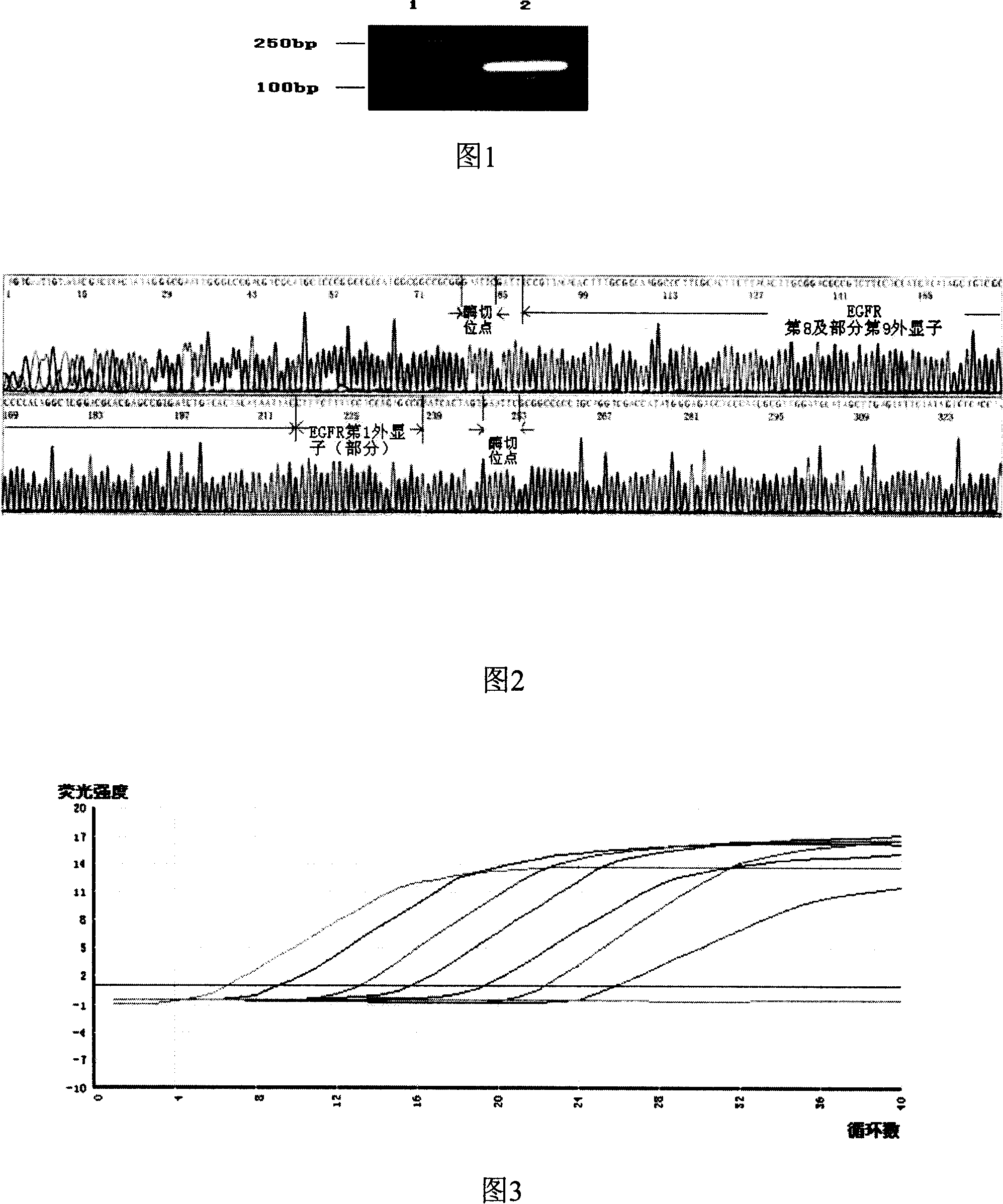
[0042] Taking the EGFR genome sequence (Genbank accession number: AY588246) as a reference, EGFRvIII is the splice variant form of the deletion of the 2nd to 7th exons. Primers were designed to amplify the 1st exon (Exon1, E1) and the 8th to part of the 9th exon sequence ...
Embodiment 2
[0063] Application of Fluorescence Quantitative PCR to Detect EGFRvIII
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com