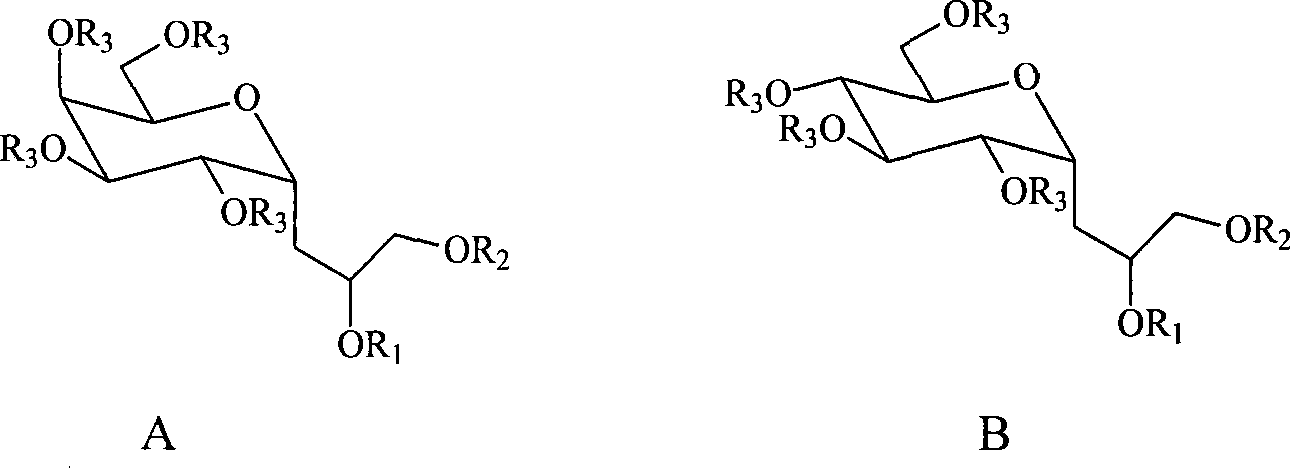
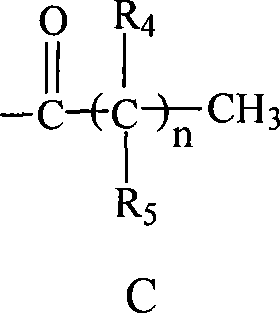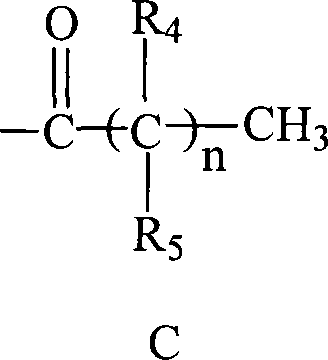C-glycosides type slycolipid compounds and use thereof
A carbon glycoside glycolipid compound technology, applied in the field of carbon glycoside glycolipid compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Add compound 1 (3.55g, 9.49mmol), anhydrous acetonitrile 50mL, trimethylallyl silicon (3.82mL, 28.62mmol) into a 100mL single-necked flask, add BF dropwise with a constant pressure funnel under ice-water bath conditions 3 .Et 2 O (6.3 mL, 47.70 mmol). Stir for seven days (react for about one day and the solution turns orange-red, and the color gradually deepens as time goes on). After the reaction is completed with thin-plate chromatography TLC tracking, the reaction solution is slowly poured into saturated sodium bicarbonate solution and stirred, and there are a lot of bubbles Overflow, extract with 50mL×3 of 1,2-dichloroethane, dry the organic phase with anhydrous sodium sulfate overnight, filter, distill off the solvent under reduced pressure, and finally separate the product by column chromatography, the eluent is petroleum ether: ether =1:1 (v / v), to obtain syrupy compound 2 (1.50g), yield 42.6%.
[0031]
[0032] 0.1716g of compound 2 was dissolve...
Embodiment 2
[0040]
[0041] Dissolve 256.6 mg of compound 3 in 17 ml of anhydrous dichloromethane, add 719.2 mg of stearic acid, 9 mg of DMAP, and stir vigorously. After the solid is fully dissolved, add 79.2 mg of DCCl and react at room temperature for 4 to 5 hours. TLC traces the complete reaction of the substrate , filter under reduced pressure with a funnel, filter out the solid, take the filtrate, and distill under reduced pressure to obtain a fatty solid. Separation by column chromatography and gradient washing with a mixed solution of petroleum ether and ethyl acetate (v / v=5 / 1-4 / 1) as eluents gave compound 6 (141.2 mg) as a fatty solid in a yield of 23.8%.
[0042] h 1 -NMR (500MHz, CDCl3) δ 5.37-5.36 (d, J=3.0Hz, 1H, H-4), 5.22-5.19 (dd, J=5.0, 10.0Hz, 1H, H-2), 5.15-5.12 (m, 2H, H-3, H-2'), 4.31-4.28 (dd, J=3.5, 12.0Hz, 1H, H-6a), 4.26-4.20 (m, 2H, H-1, H-3 'a), 4.16-4.13 (dd, J=6.0, 12.0Hz, H-3'b), 4.08-4.02 (m, 1H, H-5), 4.01-3.98 (dd, J=5.5, 12.0Hz, 1H, h-6b), 2.29-2.23(...
Embodiment 3
[0047] Antitumor activity test
[0048] The inhibition of the proliferation of HeLa cells (purchased from the Cell Bank of Chinese Academy of Sciences) was determined by the MTT method. Cells in the logarithmic growth phase were diluted to 2×104cell / ml and seeded in 96-well plates. After adhering to the wall, culture solutions containing different concentrations of drugs (25, 50, 100ug / ml) were added, and the control group was cultured with equal volumes. For each concentration, 3 parallel holes were cultured in a 37°C, 5% CO2 incubator for 24 hours, then the supernatant was discarded, and 200uL of medium containing MTT (final concentration 0.5g / L) was added to each well, and the culture was continued for 4 hours, and then the supernatant was discarded. Discard the supernatant, add 100uL of DMSO to dissolve the precipitate. The optical density (OD) value was measured with an enzyme-linked immunosorbent assay (Bio-Rad 550) with a wavelength of 492 nm and a reference wavelength...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com